Translate this page into:
1 Venkat Charmalaya – Centre for Advanced Dermatology and Postgraduate Training (RGUHS), Bengaluru, Karnataka, India
2 Department of Dermatology, Vikram Hospital, Mysuru, Karnataka, India
3 Department of Dermatologist, Inlaks and Budhrani Hospital, Pune, Maharashtra, India
Correspondence Address:
Venkataram Mysore
Venkat Charmalaya – Centre for Advanced Dermatology and Postgraduate Training (RGUHS), 3437 1 G Cross, 7 Main, Subbanna Garden, Vijay Nagar, Bengaluru - 560 040, Karnataka
India
|
How to cite this article: Mysore V, Omprakash H M, Khatri GN. Isotretinoin and dermatosurgical procedures. Indian J Dermatol Venereol Leprol 2019;85:18-23 |
Copyright: (C)2019 Indian Journal of Dermatology, Venereology, and Leprology
Abstract
Several early reports suggested that performance of dermatosurgical procedures in patients on oral isotretinoin is associated with abnormal skin healing, keloid or hypertrophic scar formation. However, this association has been recently questioned in some studies. This review examines this issue, analyzes the studies published and concludes that the recommendation made earlier about the need to avoid dermatosurgical procedures in patients on isotretinoin is based on inadequate and insufficient evidence and hence needs revision. The review also suggests that recent studies on the subject establish that performing such procedures is safe.
Keywords: Hypertrophic scar, isotretinoin, keloid, lasers, surgery
Introduction
“Isotretinoin is a godsend for patients with scarring acne,” remarked Steven R Feldman, in his editorial comment.[1] Isotretinoin, a retinol derivative of vitamin A, is widely used in the treatment of acne vulgaris and has many pharmacological actions that affect epidermis, sebaceous gland and collagen formation.[2],[3],[4] The propensity of isotretinoin to affect these functions has led to questions about the possibility of poor wound healing, development of keloid and hypertrophic scarring, particularly in patients who undergo dermatosurgical procedures while on this drug.[5] This has led to statutory recommendation that cosmetic and dermatosurgical procedures should be avoided during and after administration of the drug for 6–12 months.[6],[7],[8],[9],[10] This recommendation has been debated, and differences of opinion exist on the subject.[11],[12],[13]
This study reviews the current literature on the subject to examine whether the recommendation is based on evidence and if a modification is justified.
Literature Search
A PubMed search was done on 9 October, 2016 using keywords “isotretinoin,” “isotretinoin side effects,” “isotretinoin” AND “laser,” “isotretinoin” AND “surgery,” “isotretinoin” AND “keloid,” “isotretinoin” AND “would healing,” “isotretinoin” AND “hypertrophic scarring.” A total of 403, 62 and 27 articles were found with respect to “isotretinoin” AND “surgery,” “isotretinoin” AND “wound healing” and “isotretinoin” AND “laser,” respectively. Of these, 26 articles in English language, which consisted of reports and studies with respect to isotretinoin and procedures, were considered to be relevant and included. The studies can be analyzed under two categories:
- Early studies that suggested is a tendency for poor wound healing, development of keloids and hypertrophic scars in patients on isotretinoin, either spontaneously or after dermatosurgical procedures
- More recent studies that seem to suggest that such a tendency is not commonly seen.
Several early studies have documented occurrence of keloids spontaneously during administration of isotretinoin.[14],[15] Ginarte et al. documented “spontaneous occurrence” of keloid on trunk in a 16-year-old boy, who was started on 0.5 mg/kg/day oral isotretinon.[15] The keloid occurred within 4 weeks after starting the medication and continued to progress despite stopping isotretinoin. On follow-up at 2 years, keloids persisted despite administration of intralesional triamcinolone. Dogan (2006) reported a patient, who had no previous history of keloid, but developed truncal keloid after 8 weeks of initiating oral isotretinoin 40 mg/day.[16] In a study of 21 patients by Guadanhim et al., spontaneous occurrence of keloid was documented in a single case.[14]
Subsequently, few studies also reported keloids in patients on isotretinoin who underwent dermatosurgical procedures. Zachariae (1988) reported three cases.[17] First case underwent argon laser on the cheek. This case developed keloid after 8 weeks. The other two cases underwent dermabrasion and developed postprocedure keloids at 8 weeks and 6 months. Rubenstein et al. presented a case series of 6 patients who underwent dermabrasion for acne scarring, 3 while still taking isotretinoin and 3 who had stopped taking isotretinoin 5–6 months previously.[18] The dose of isotretinoin was 0.5–1 mg/kg/day with duration of treatment varying from 4 to 14 months. All of these patients developed keloid scars 1–3 months after dermabrasion. Topical and intralesional corticosteroids resulted in some clinical improvement or resolution of the keloids.
The above studies, though small, underlined the possibility of development of keloids in patients on isotretinoin patients and subsequently lead to the recommendation that procedures should not be performed in such patients for 6–12 months. However, as can be seen, the studies were few, had a small sample size, many were case reports and all were reported at a time when lasers were in their early stage of development. The adverse effects developed on performance of aggressive procedures such as argon laser, and dermabrasion, which are rarely performed in today's practice. The recommendation also applied to all procedures, irrespective of the invasiveness (or the lack of it). The profile of procedures have changed over time with more and more minor procedures, such as microdermabrasion, superficial peels and microneedling, being performed routinely. Laser machines have become safer, more precise and also less invasive. Hence it is interesting to examine the more recent studies, about each individual procedural intervention in patients on isotretinoin.
Dermabrasion
It is interesting to note that in contrast to the above studies documenting development of keloids, several recent studies have documented the safety of this procedure in this patient group. As early as 1985, Roenigk et al. documented a case series of 9 patients of acne scarring who underwent full-face dermabrasion.[19] Out of these 9 patients, 4 had discontinued isotretinoin 2–11 months previously and 5 were either still taking isotretinoin at the time of undergoing dermabrasion. The mean isotretinoin dosage for all patients was 0.5–1 mg/kg/day. Normal wound healing was noted in all patients. Bagatin et al. documented a prospective cohort study of 7 patients (six female, 1 male), who had been taking isotretinoin for a minimum of 1 month, with a dose range of 10–40 mg/day.[20] On each patient, 1 cm2 area of depressed scarring was selected and manual dermabrasion with a diamond fraise was performed. No complications including hypertrophic or keloid scarring were documented in these patients during follow-up period of 6 months. Picosse et al. reported the outcomes in 10 patients (4 female, 6 male) who underwent medium-depth chemical peel with Jessner solution and 35% trichloroacetic acid, of the entire face, followed by manual dermabrasion with sterile sandpaper for scarred area.[21] All the patients had completed treatment with isotretinoin for their acne 1–3 months previously, with a minimum accumulated dose of 122 mg/kg per patient. No evidence of hypertrophic or keloidal scarring was found in any of the treated patients. In a more recent study, Mahadevappa et al. performed fractional Er:YAG laser resurfacing (which is the laser equivalent of motor dermabrasion) on face, in a case with keloid on chest with concomitant intake of isotretinoin.[22] Postoperative follow-up of year did not show any keloid on face and the preexisting, chest keloid remained unaffected.
It is somewhat difficult to speculate why earlier studies with dermabrasion should document keloids in contrast to later studies. Such cases may represent idiosyncratic response to oral isotretinoin. However, as stated earlier, dermabrasion is rarely practiced now, and hence it is perhaps a situation which would be rarely encountered in current practice.
Laser Hair Removal
[Table - 1] shows different studies on laser hair removal and their adverse events profile. Khatri (2004) performed diode hair reduction in 7 patients, taking concomitant isotretinoin.[23] No side effects including keloids were reported at 1 month. Cassano et al. (2005) performed diode hair reduction on face in 6 patients, taking concomitant isotretinoin.[24] Again no side effects including keloids were reported, in a follow-up period of 4 years. In a later study in 2006, Khatri and Garcia used long-pulsed flashlight for hair reduction in 6 patients on isotretinoin, on both facial and extrafacial areas.[25] Again here no keloid was reported in a follow-up of 6 months. Khatri in another study in 2009 performed facial and extrafacial hair reduction in 11 cases with long-pulsed Nd:YAG, taking concomitant isotretinoin.[26] At 12 weeks follow-up, no keloid was reported. Chandrashekar et al. (2014) published a retrospective study of hair reduction in 5 cases.[27] The devices used were diode 980 long-pulsed Nd:YAG 1064 nm and intense pulse light and follow-up period of 24 weeks. No adverse events were documented. Mahadevappa et al. (2016) performed 26 sessions of hair reduction safely, of which 9 were intense pulse light, 4 of long-pulsed Nd:YAG laser and 13 of diode laser.[22] The follow-up period was 1 year and no keloids were reported.
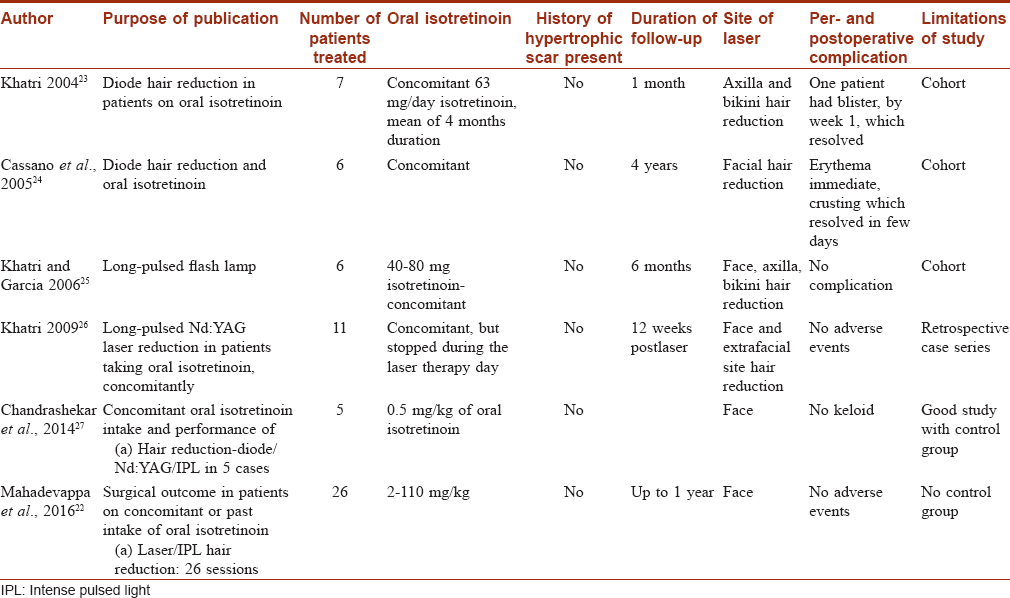
Thus these studies show that hair reduction with lasers of all wavelengths and intense pulse light systems is safe in patients on isotretinoin.
Lasers for Postacne Scarring [Table - 2]
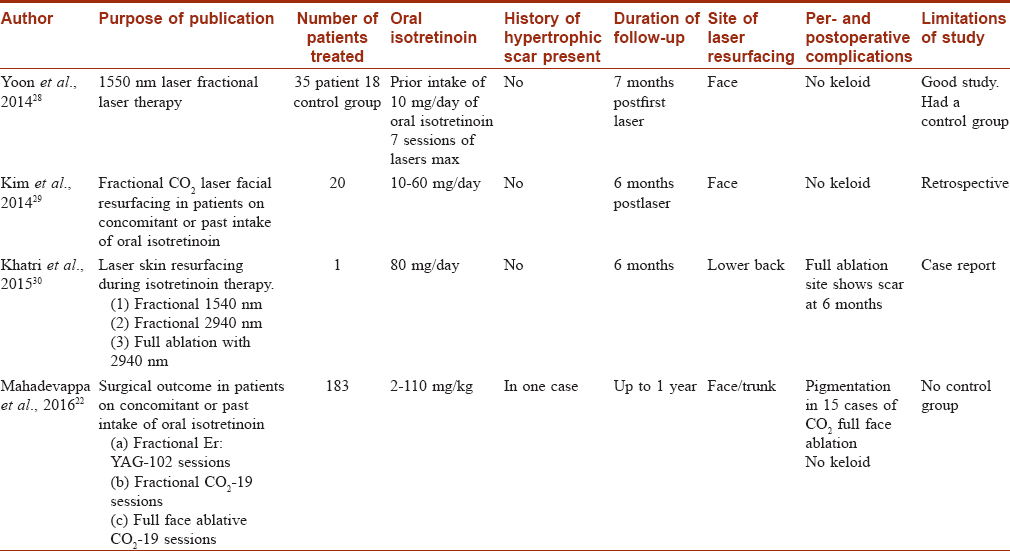
Ablative, fractional ablative and nonablative lasers all affect neo collagen synthesis and are used for the management of postacne scarring. Four papers in PubMed since 2014 are been reviewed in [Table - 2].
Yoon et al. (2014) performed 1550 nm, infrared laser therapy in 35 patients on isotretinoin, for treatment of acne scarring.[28] The control group included 18 patients, not on isotretinoin. A mean of 3.1 laser sessions were performed in both groups. Both the groups did not show any keloids or hypertrophic scars. The retrospective study by Chandrashekar et al. (2014) included 25 cases (on 0.5 mg/kg/day isotretinoin) and a control group of 25 cases who were not on isotretinoin.[27] Both groups underwent fractional CO2 laser treatments. Follow-up of 24 weeks did not show any keloids or hypertrophic scars in either groups. Kim et al. (2014) performed fractional CO2 laser on face, in a retrospective study in 20 cases on 10–60 mg/day of isotretinoin.[29] Follow-up at 6 months did not show any side effects. Mahadevappa et al. did a prospective study in 183 patients taking isotretinoin in the dose range of 2–110 mg/kg.[22] Laser-assisted facial resurfacing (both by conventional full-face resurfacing and fractional CO2 and Er:YAG lasers) was performed in these patients in a total of 45 patients, with a follow-up of 1 year. None of the patients developed hypertrophic scar or keloid, despite being on isotretinoin. Khatri et al. treated the lower back with three different lasers in a patient, who was on 80 mg/day isotretinoin.[30] At 6 months, there was no evidence of any hypertrophic scarring.
Thus the studies using conventional full face and fractional lasers for postacne scarring seem to suggest the lack of adverse events and the safety of these procedures in patients on isotretinoin.
Isotretinoin and Chemical Peeling
Chemical peels are performed for correction of scars, reduction of pigmentation and treatment of active acne and for photoaging of skin. Superficial peels are usually used and there have been several studies documenting the use of peels in patients on isotretinoin. There have been no reports of deep peels in patients on isotretinoin, perhaps because of lack of use of deep peels in current practice.
Picosse et al. performed 10 medium depth peels with Jessner's and 35% trichloracetic acid, followed by sand paper dermabrasion.[21] No keloid was reported, despite 3 cases having keloid prior to peels. The study conducted by Mahadevappa et al. included trichloroacetic acid peel in 4 sessions, salicylic acid peel in 30 sessions, combination peels in 65 sessions and lastly glycolic acid peel in 147 sessions.[22] There was only one report of one keloid in a case of glycolic acid peel: interestingly on chest, a site distant from peel. While the safety of different peels seemed to be established by these studies, the relevance of the development of keloid on chest to the peel performed on face could not be explained. A case of severe hyperpigmentation and scarring following 70% glycolic acid peel was reported in 2014 by Gerber et al. in a 34-year-old woman who had stopped isotretinoin 10 weeks prior to peel.[31] The authors noted that the “exact mechanism of atypical reepithelization and scarring due to isotretinoin remained unclear. Oral retinoids may cause disruption of the stratum corneum and thereby enhance the depth of penetration of the glycolic acid peel.” The hyperpigmentation which is a known side effect of peels may also be due to enhanced sensitivity to sunlight after the peel in a patient with thinned skin due to isotretinoin. This report of hyperpigmentation with scarring suggests that caution needs to be exercised while performing glycolic acid peels in these patients, even though other peels such as salicylic acid peels seem to be safe.
Isotretinoin and Radiofrequency Procedure
Radiofrequency device is used for cutting, coagulating or ablation of skin lesion. In addition, it is used for collagen remodeling by using dermal needles or epidermal bipolar or tripolar electrodes. Chandrashekar et al. performed 13 cases of acne scar revision using microneedling radiofrequency in patients on 0.5 mg/kg of oral isotretinoin.[27] None developed any complications. In the study conducted by Mahadevappa et al., radiofrequency ablation of compound nevus was done in a patient with cumulative dose of isotretinoin 4000 mg, and this patient later developed a keloid at that site.[22] The precise cause could not be established.
Isotretinoin and Other Procedures
Other common procedures performed in dermatology include skin biopsies, excision, comedones extraction, punch elevation of acne scars, microneedling for scars, microdermabrasion and subcision. Mahadevappa et al. had 27 such interventions in 11 cases.[22] None developed complications. This study also included 18 patients who underwent 44 sessions of microdermabrasion with aluminum crystals, on face. No side effects were documented. In the study conducted by Chandrashekar et al., 12 cases of microneedling in isotretinoin group was compared with 12 cases in control group.[27] At 24 weeks no keloid or scarring was noted.
Should the guidelines of avoiding surgery in patients taking isotretinoin be modified?
The above review demonstrates, rather overwhelmingly, that barring a few studies in a small number of patients, the risk of hypertrophic scarring, keloid formation, delayed wound healing are not seen in most dermatological procedures. As such, the recommendation to avoid procedures in patients who have been administered isotretinoin, in our opinion, was based on inadequate evidence and of poor relevance to today's practice:
- The studies on which such a recommendation was based on were few, of low quality, with a small number of patients. The evidence could be classified as level 3 and grade of recommendation as level D (Harbour and Miller's revised grading system)
- Further, those studies that were documented in early 1980s and 1990s were based on aggressive procedures such as dermabrasion, which are rarely performed today
- The recommendation was made at a time when laser technology and other minor procedures such as superficial peels and microdermabrasion were yet to develop fully. It needs to be emphasized also that procedures are far less invasive in today's practice and application of such a recommendation to these minimally invasive procedures needs to be questioned
- Hence the relevance of such data needs to be questioned and the authors are of the firm opinion that such a recommendation based on such poor quality reports would never have been accepted in today's standards of evidence-based practice, had it been made. The authors feel that the recommendation has hindered the use of the procedures in such patients, and fear in the minds of physicians about potential medicolegal situations. This was borne out by a recent survey of nationally recognized experts in laser surgery regarding the treatment of patients taking or within 6 months of isotretinoin therapy. In this report, maximum number of respondents (70%) affirmed that medical-legal concerns guided their decision-making regarding this patient population, in contrast to concerns about atypical or poor wound healing (69%), scarring (66%), and about hypertrophic or keloidal scarring (49%). What was interesting was 76% of the respondents had never seen any complications in their own clinical practice[32]
As such, the recommendation has only served to deny patients of safe treatments and put unnecessary fear in the minds of doctors. As this review shows, most procedures in current practice are less invasive, and the recent studies reviewed here, though admittedly few and small, suggest the relative safety of the procedures in this group of patients. The time has come therefore to put in a new recommendation on this issue. A recent consensus guidelines publication based on a systematic review of 32 relevant publications involving 1485 procedures showed that there was insufficient evidence to support delaying manual dermabrasion, superficial chemical peels, cutaneous surgery, laser hair removal and fractional ablative and nonablative laser procedures for patients currently receiving or having recently completed isotretinoin therapy.[33] The publication further stated that mechanical dermabrasion and fully ablative laser are not recommended in the setting of systemic isotretinoin treatments. The review suggested that physicians and patients should have an evidence-based discussion regarding the known risk of cutaneous surgical procedures prior to performing them in the setting of systemic isotretinoin therapy. For some patients and some conditions, an informed decision may lead to earlier and potentially more effective interventions.
The authors entirely agree with the above opinion and feel that the recommendation is out of place in current practice in view of the available current evidence and needs to be withdrawn. The authors would also suggest that a test treatment patch be performed, when necessary, in selected patients, at the discretion and judgement of the physician, instead avoiding or delaying the procedures.
Summary
The authors recommend that the recommendation for avoiding procedures such as fractional CO2 resurfacing, fractional Er:YAG laser, fractional infrared lasers-1550 nm, 1540 nm, laser hair removal, microdermabrasion using aluminum crystals, microneedling, microneedling with radiofrequency, cold steel surgeries such as subcision, skin biopsies, in patients on isotretinoin, is based on poor and limited evidence. Instead of avoiding the procedures, a test procedure may be performed to establish safety in selected patients at the discretion of the treating physician.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 2. | American Academy of Dermatology Position Statement on Isotretinoin (Approved by the Board of Directors December 9, 2000; Amended by the Board of Directors March 25, 2003, March 11, 2004, and November 13, 2010). Available from: http://www.aad.org/Forms/Policies/Uploads/PS/PS-Isotretinoin. Pdf. [Last accessed on 2016 Oct 12]. [Google Scholar] |
| 3. | Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, editors. Acne Vulgaris. London: Informa Healthcare; 2011. p. 134-45. [Google Scholar] |
| 4. | Blomhoff R, Blomhoff HK. Carotenoids and Vitamin A to retinoids. In: Vahlquist A, Duvic M, editors. Retinoids and Carotenoids in Dermatology. USA: Informa Healthcare; 2007. p. 1-25. [Google Scholar] |
| 5. | Goihman-Yar M. Keloid formation induced by isotretinoin therapy. Int J Dermatol 1999;38:228-9. [Google Scholar] |
| 6. | Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM085812.pdf. [Last accessed on 2016 Oct 12]. [Google Scholar] |
| 7. | Available from: https://www.medicines.org.uk/emc/PIL.15643.latest.pdf. [Last accessed on 2016 Oct 12]. [Google Scholar] |
| 8. | Lovell CR. Keloids and hypertrophic scars. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed., Ch. 96. Oxford, UK: John Wiley and Sons Ltd.; 2016. p. 96.45-96.46. [Google Scholar] |
| 9. | Habif TP, editor. Acne, rosacea and related disorders. In: Clinical Dermatology. 6th ed., Ch. 7. Philadelphia, USA: Elsevier Inc.; 2016. p. 247-8. [Google Scholar] |
| 10. | Zaenglein AL, Thiboutot DM. Acne vulgaris. In: Bolognia JL, Jurizzo JL, Schaffer JV, editors. Dermatology. 3rd ed., Ch. 36. Philadelphia, USA: Elsevier, Saunders; 2012. p. 557-8. [Google Scholar] |
| 11. | James WD, Elston DM, Berger TG, editors. Acne vulgaris. In: Andrew's Diseases of the Skin-Clinical Dermatology. 12th ed., Ch. 13. Philadelphia, USA: Elsevier Inc.; 2016. p. 230-1. [Google Scholar] |
| 12. | Pattan TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, editor. Comprehensive Dermatology Drug Therapy. 3rd ed., Ch. 20. Philadelphia, USA: Elsevier Saunders; 2013. p. 252-68. [Google Scholar] |
| 13. | Zouboulis CC, Bettoli V. Management of severe acne. Br J Dermatol 2015;172 Suppl 1:27-36. [Google Scholar] |
| 14. | Guadanhim LR, Gonçalves RG, Bagatin E. Observational retrospective study evaluating the effects of oral isotretinoin in keloids and hypertrophic scars. Int J Dermatol 2016;55:1255-8. [Google Scholar] |
| 15. | Ginarte M, Peteiro C, Toribio J. Keloid formation induced by isotretinoin therapy. Int J Dermatol 1999;38:228-9. [Google Scholar] |
| 16. | Dogan G. Possible isotretinoin-induced keloids in a patient with Behçet's disease. Clin Exp Dermatol 2006;31:535-7. [Google Scholar] |
| 17. | Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol 1988;118:703-6. [Google Scholar] |
| 18. | Rubenstein R, Roenigk HH Jr., Stegman SJ, Hanke CW. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol 1986;15:280-5. [Google Scholar] |
| 19. | Roenigk HH Jr., Pinski JB, Robinson JK, Hanke CW. Acne, retinoids, and dermabrasion. J Dermatol Surg Oncol 1985;11:396-8. [Google Scholar] |
| 20. | Bagatin E, dos Santos Guadanhim LR, Yarak S, Kamamoto CS, de Almeida FA. Dermabrasion for acne scars during treatment with oral isotretinoin. Dermatol Surg 2010;36:483-9. [Google Scholar] |
| 21. | Picosse FR, Yarak S, Cabral NC, Bagatin E. Early chemabrasion for acne scars after treatment with oral isotretinoin. Dermatol Surg 2012;38:1521-6. [Google Scholar] |
| 22. | Mahadevappa OH, Mysore V, Viswanath V, Thurakkal S, Majid I, Talwar S, et al. Surgical outcome in patients taking concomitant or recent intake of oral isotretinoin: A multicentric study-ISO-AIMS study. J Cutan Aesthet Surg 2016;9:106-14. [Google Scholar] |
| 23. | Khatri KA. Diode laser hair removal in patients undergoing isotretinoin therapy. Dermatol Surg 2004;30:1205-7. [Google Scholar] |
| 24. | Cassano N, Arpaia, N, Vena GA. Diode laser hair removal and isotretinoin therapy. Dermatol Surg 2005;31:380-1. [Google Scholar] |
| 25. | Khatri KA, Garcia V. Light-assisted hair removal in patients undergoing isotretinoin therapy. Dermatol Surg 2006;32:875-7. [Google Scholar] |
| 26. | Khatri KA. The safety of long-pulsed Nd: YAG laser hair removal in skin types III-V patients during concomitant isotretinoin therapy. J Cosmet Laser Ther 2009;11:56-60. [Google Scholar] |
| 27. | Chandrashekar BS, Varsha DV, Vasanth V, Jagadish P, Madura C, Rajashekar ML, et al. Safety of performing invasive acne scar treatment and laser hair removal in patients on oral isotretinoin: A retrospective study of 110 patients. Int J Dermatol 2014;53:1281-5. [Google Scholar] |
| 28. | Yoon JH, Park EJ, Kwon IH, Kim CW, Lee GS, Hann SK, et al. Concomitant use of an infrared fractional laser with low-dose isotretinoin for the treatment of acne and acne scars. J Dermatolog Treat 2014;25:142-6. [Google Scholar] |
| 29. | Kim HW, Chang SE, Kim JE, Ko JY, Ro YS. The safe delivery of fractional ablative carbon dioxide laser treatment for acne scars in Asian patients receiving oral isotretinoin. Dermatol Surg 2014;40:1361-6. [Google Scholar] |
| 30. | Khatri KA, Iqbal N, Bhawan J. Laser skin resurfacing during isotretinoin therapy. Dermatol Surg 2015;41:758-9. [Google Scholar] |
| 31. | Gerber PA, Kukova G, Bölke E, Homey B, Diedrichson E. Severe hyperpigmentation and scarring following glycolic acid peel treatment in combination with low-dose isotretinoin. Eur J Med Res 2014;19:60. [Google Scholar] |
| 32. | Prather HB, Alam M, Poon E, Arndt KA, Dover JS. Laser safety in isotretinoin use: A survey of expert opinion and practice. Dermatol Surg 2017;43:357-63. [Google Scholar] |
| 33. | Spring LK, Krakowski AC, Alam M, Bhatia A, Brauer J, Cohen J, et al. Isotretinoin and timing of procedural interventions: A systematic review with consensus recommendations. JAMA Dermatol 2017;153:802-9. [Google Scholar] |






































