OMIC Underwriting Manager
Digest, Summer 1999
Technological advances, demographic shifts, and socioeconomic forces over the past decade have brought about profound changes in the practice of ophthalmology and the resulting liability exposure. Through the years, OMIC has responsibly modified its coverage features and liability limits to meet these changes.
Refractive Surgery
Until 1995, virtually the only surgical option for treatment of myopia was radial keratotomy (RK). Then in 1995, the FDA approved the Summit laser for photorefractive keratectomy (PRK). Since that time, LASIK has essentially become the procedure of choice for many. Meanwhile, several other refractive surgery options have emerged, and OMIC has extended coverage to insureds who perform these procedures: RK/AK, PRK, ALK, LASIK, Intacs, Clear Lens Extractions, and Phakic Implants for refractive purposes.
In the past year, OMIC has modified at least six existing guidelines for refractive surgery and developed guidelines for Intacs. Recent policy modifications include:
The maximum degree of myopia for PRK and LASIK was increased in recognition of changes in FDA-approved guidelines.
The minimum age for PRK using the Summit laser was lowered to 18.
The required one-week interval between primary PRK and LASIK procedures may be waived for experienced surgeons who qualify, subject to special consent requirements.
The 10 surface PRK requirement was discontinued to allow physicians with limited or no prior PRK experience to qualify for LASIK coverage provided they are certified on the laser and are proctored for their first five LASIK cases.
Cosmetic Surgery
Many ophthalmologists have expanded the scope of their practice to include laser skin resurfacing, laser hair removal, and other cosmetic surgery procedures, including facelifts and liposuction. Subject to underwriting review and adherence to guidelines, coverage for such cosmetic procedures is now available to qualified ophthalmologists.
Part-time and Tail Coverage
While OMIC has always offered part-time rates to ophthalmologists who limit their practice to medical ophthalmology, recently this discount was extended to qualified insureds who perform surgery as well. Eligibility for a part-time discount is based upon the number of practice hours, scope and volume of practice, and claims history, among other things.
Recognizing that an increasing number of ophthalmologists may elect to retire at an earlier age, OMIC discontinued its age requirement for free tail coverage upon retirement and, more recently, reduced the length-insured requirement. Now, an insured who retires permanently from the practice of medicine at any age qualifies for a free tail provided he or she has been continuously insured with OMIC for at least one year (see note below). OMIC also provides a free tail upon death or disability, regardless of age or length of time insured with OMIC.
Liability Limits
Legislative changes have prompted requests for more flexible coverage options in certain states. OMIC now offers limits of $400,000 per claim/$1,200,000 aggregate to insureds who practice in Pennsylvania; $250,000/$750,000 in Indiana; and $1,500,000/$4,500,000 in Virginia. OMIC also considers, on a case-by-case basis, requests by insureds for non-traditional limits. (Note: the limits noted here applied in 1999 – limits in PA and VA have increased since this article was written)
New Entity Exposures
OMIC realized the need to develop guidelines accommodating the liability exposures associated with shared management of post-operative patients. OMIC will directly insure qualified employed optometrists as additional insureds under the physician’s policy and, in certain limited circumstances, may extend coverage to the entity and/or optometrist in cases where the optometrist owns a portion of the practice.
To remain financially viable, ophthalmic surgery centers often must allow other specialists to use their facility. Thus, OMIC has developed appropriate underwriting guidelines and rates that permit the company to insure the surgery center for its vicarious liability exposures arising from ophthalmic and non-ophthalmic procedures. At the request of an insured network, OMIC also developed a program for coverage of qualified eye banks.
Fraud and Abuse Coverage
In response to the federal government’s zealous efforts to root out health care fraud and abuse, OMIC now provides its professional liability insureds with a Medicare/Medicaid Fraud and Abuse Legal Expense Reimbursement Insurance policy free of charge. Academy members insured elsewhere for their professional liability coverage may purchase fraud and abuse coverage from OMIC for a nominal premium.
For more information or to request changes to your coverage, please contact any of OMIC’s Underwriting Department representatives at (800) 562-6642. For information regarding OMIC’s fraud and abuse coverage, please contact Kim Wittchow at ext. 653.
UPDATED (1/13/2013): OMIC’s retirement premium waiver was modified in 2003 to increase the required minimum period of continuous insurance from 1 year to 5 years for all new policies incepted on or after 6/1/2003 and for all individual insureds added to existing group policies on or after 6/1/2003.
Coverage Issues // No Comments
By Michael Meyers
Mr. Meyers is a freelance writer in Charlottesville, VA. He has written extensively about the insurance industry.
[Digest, Fall 1999]
Most doctors and other professionals would never dream of going without life, auto, homeowners, and medical insurance for their families. Yet they risk the assets they’ve worked so hard to build up by overlooking another equally important personal insurance protection: long-term care insurance. They often believe that a nursing home represents a loss of independence, that it is the only form of long-term care available, and that long-term care insurance is too expensive.
Nursing homes are not the only long-term care option. More and more people are taking advantage of assisted-living facilities, senior housing with services, adult foster care, home health care, and other innovative forms of long-term care to avoid the high cost of a nursing home stay. According to the Health Care Financing Administration, the average annual cost of a nursing home stay is $47,000 and rising. Of course, the cost of alternative services can add up quickly too, and even a single year of long-term care paid for out-of-pocket can be more costly than insurance.
Changes in federal income tax incentives in 1997 allowed qualified long-term care insurance premiums to be deducted as medical expenses, in whole or in part, subject to certain limits, provided the plan meets federal guidelines. Employers who pay premiums for an employee and/or spouse can deduct them as a business expense. Solo practitioners or doctors in a partnership, including S corporations, may deduct long-term care premiums to the same extent they do health insurance premiums.
The younger a person is at the time long-term care insurance is purchased, the lower the premium. Many people wait too long to buy this coverage. Not only does this raise the premium, it increases the likelihood of developing a chronic illness that will disqualify the applicant for coverage. When evaluating the need for long-term care insurance, take into account the individual’s personal situation, retirement plans, and family relationships. Ask yourself the following questions:
How Long Should Coverage Last?
Given the statistics on lengths of nursing home stays, you probably want to shop for a long-term care policy that provides two to four years of care. More coverage may be a waste, less a big risk.
How Do I Decide on a Daily Benefit Amount?
Research the costs of nursing home care where you live, keeping in mind that it is likely you will need to pay a nursing home more than the basic rate each month because of added charges for drugs and special services. One approach is to select a daily benefit equal to the nursing home’s published rate and plan to pay out-of-pocket for extra services. You also may want to take into account your projected discretionary income and how much you will be able to afford to pay out-of-pocket.
How Much Can I Afford Out-of-Pocket?
Even with this insurance, you will have to pay some costs yourself. First, you must meet a deductible (elimination period). Then, some plans pay a fixed amount for each day you are in a nursing home, regardless of how much your nursing home care cost, while other plans pay actual charges up to a fixed amount. How a plan pays its benefits will influence how large a benefit you select, so factor this into your decision.
What Coverage Benefits Should I Look For?
The company behind the coverage. The carrier’s financial stability and experience providing long-term care insurance are the first things to consider. You want assurance that the company will be around in ten or fifteen years if you need to apply for benefits. A.M. Best, Moody’s, or Standard & Poor’s rating systems can help you judge.
Type of coverage provided.
Look for a plan that covers skilled, intermediate, and custodial nursing home care.
Home health care.
You’ll most likely want a plan that offers home health care as an option or as one of its regular benefits in situations where nursing home care is not necessary.
Definition of a covered stay.
A good plan should cover your nursing home stay not only if you are injured or sick but also if you need continual help with two “activities of daily living” (i.e., dressing, toileting, continence, transferring, or feeding) or you need supervision because of cognitive impairments like Alzheimer’s Disease.
Choice, choice, choice.
Different people have different needs based on their income and assets, nursing home costs where they live, and how much they can or want to “self-insure.” Select a plan that gives you a choice of daily benefit options, elimination periods (deductibles), and benefit limits. That way you can build a plan to meet your individual needs.
Inflation protection.
Because you are purchasing insurance today to meet tomorrow’s costs, you probably will want inflation protection. There are two types of inflation protection available: one automatically increases your benefit level by an equal percentage each year; the other compounds the benefit increases.
Guaranteed renewable.
This means you can always renew your coverage as long as you pay your premiums on time, even if your health condition worsens. In most cases, however, the insurer will reserve the right to change premiums for all persons in a given class or state.
Waiver of premium.
This allows you to continue coverage without cost after you have been receiving benefits for a certain period of time, such as 90 days. Some plans offer a “return of premium” or nonforfeiture benefit in case you die or terminate your coverage. However, this provision can add significantly to the cost of a plan.
If you need additional help shopping for long-term care insurance, contact the National Association of Insurance Commissioners at (816) 374-7259 for a copy of the Shoppers Guide to Long-term Care.
The American Academy of Ophthalmology offers comprehensive long-term care plans to active Academy members and their spouses, parents, and grandparents. To learn more about these plans, call the Academy Insurance Center at (800) 906-7607 for a free consultation.
Coverage Issues, Policy Issues // No Comments
By Betsy Kelley
OMIC Underwriting Manager
[Digest, Spring 2000]
Contracts are a fact of life in most ophthalmic practices today. Providers sign contracts with health care plans, laser centers, finance companies, and other entities. Contracts outline each party’s responsibilities, compensation, and other terms. In many instances, contracts may include provisions that affect a provider’s professional liability exposure.
One provision frequently found in contracts is a hold harmless or indemnification clause whereby one party (usually the physician) agrees to contractually assume the liability exposure of the other party. Some indemnification clauses are quite broad, requiring that the physician hold the other party harmless for “any and all claims, suits, losses, or damages” arising from services rendered, without regard to which party was responsible for such activities or whether negligence was involved.
Other clauses are sufficiently narrow and require that the physician hold the other party harmless only for “claims, suits, losses, or damages arising solely from the physician’s negligence and not otherwise covered by insurance.” Indemnification clauses may be unilateral, meaning that only one party holds the other harmless, or they may be mutual, meaning that both parties agree to hold the other harmless for its own negligent actions.
OMIC provides limited contractual liability coverage within policy limits for indemnity and reasonable defense costs that insureds become legally obligated to pay pursuant to a hold harmless or indemnification agreement in a written contract between the insured and a hospital, health maintenance organization, preferred provider organization, or other managed care entity. This coverage is limited to indemnity and defense costs incurred solely from the performance of professional services provided by the insured and is solely for medical incidents otherwise covered under the policy. In certain circumstances, OMIC may, for an additional premium, extend contractual liability coverage by endorsement to other entities that are not engaged in the practice of medicine but may incur liability exposure as a result of their relationship with the insured.
OMIC’s policy excludes coverage for liability assumed under contract with other types of organizations if such liability would not exist in the absence of the contract. Therefore, OMIC generally recommends that indemnification clauses be removed from the contract, if possible. If the clause cannot be removed, OMIC recommends that it be replaced with a narrow, mutual hold harmless clause in which each party agrees to indemnify the other for losses arising solely from the party’s negligence.
Additional Insureds
A new trend is emerging in which organizations are no longer satisfied merely having the physician contractually agree to hold them harmless in the event of a claim. Instead, they are now adding clauses to their contracts requiring that the physician name them as an additional insured under the physician’s policy.
OMIC is generally able to name a third party as an additional insured only in situations where the entity is a management services organization (MSO) involved in administrative activities such as the purchase of equipment, billing, and other matters. Because they do not render medical services themselves, such organizations are frequently unable to purchase medical malpractice policies of their own.
With the exception of MSOs, OMIC will not name third parties, such as medical professional corporations or laser refractive centers, as additional insureds. Because these organizations render professional services themselves and are likely to be responsible to some extent for supervision and control of the physician’s activities, they are equipped to insure themselves or to purchase separate coverage for their liabilities. It is generally in the best interests of both parties to purchase their own insurance policy so separate limits apply. Otherwise, each party’s limits are reduced by any indemnity paid on behalf of the other party.
Other Provisions
Other contract provisions may specify the limits of liability that the physician must carry or may require that the physician provide evidence of insurance. Upon request, OMIC can issue a certificate of insurance to the organization, specifying the insured’s policy number, effective and expiration dates of coverage, and limits of liability. In addition, OMIC also will attempt to notify the certificate holder of any material changes in coverage, such as a change in limits or cancellation of coverage. Some contracts may require that the organization be provided with advance notice prior to cancellation or coverage changes. Although OMIC will do its best to provide ample notice to certificate holders, OMIC itself may not receive sufficient advance notice of requested changes to comply with such contract provisions. For this reason, such requirements should be removed from any contract.
As a service to its insureds, OMIC will review contract language*** as it relates to professional liability issues and provide advice regarding uninsured risks. To have OMIC review these contract provisions, contact the Risk Management Department at (800) 562-6642, ext. 603 or fax the specific clauses to (415) 771-7087.
*** NOTE: OMIC no longer reviews contract language. However, as a service available only to its insureds, OMIC will provide an analysis of indemnification agreements prepared by our Legal Counsel. Policyholders may share this analysis with their own attorney. To obtain this analysis, contact the Underwriting Department at (800) 562-6642, option 1 or via email at underwriting@omic.com, or the Risk Management Department at (800) 562-6642, option 4 or via email at riskmanagement@omic.com. 10/8/14.
Coverage Issues, Policy Issues // No Comments
By James F. Holzer, JD
Mr. Holzer is OMIC’s President & CEO.
[Digest, Winter 2001]
The U.S. economy and stock market may be showing signs of renewed vibrancy this quarter, but malpractice insurance carriers are bracing for the worst. Physicians in all specialties are seeing professional liability rates skyrocket for the first time in many years. It’s a grim reminder of when so-called malpractice crises erupted in the past, driving doctors to pay higher premiums, find replacement, and seek shelter behind defensive practice patterns.
Although ophthalmologists are not immune from current adverse developments, their loss experience is better than most other specialties and, in some cases, significantly better on average than all specialties combined. OMIC’s loss experience and financial performance continues to be more favorable than the industry as a whole. Yet claims costs and related expenses require even the most conservative carrier to periodically adjust its price. A few physician-sponsored carriers have been able to keep rate increases well under the 10% this year. OMIC, for example, will adjust its premium by 7.5% for policies issued or renewed after July 1, 2001.
The news unfortunately isn’t as good for other physicians. Double-digit rate hikes are back. Many medical malpractice carriers anticipate or have already instituted premium increases that may well exceed the expected national average of 15%. Commercial (non-provider-owned) carriers seem the hardest hit with planned increases of 50% to 100% in some states. Although the relative rate for an ophthalmologist is less than say for an OB-GYN specialist, some of these large increases may apply across the board to ophthalmologists insured by these companies. One large national carrier, which has slipped from first to fifth place as the leading provider of malpractice insurance, reportedly doubled its premiums for some ophthalmologists in Arizona, Missouri, and Texas and selectively levied a 60% increase for risks in Vermont and 75% in California. Another large national provider of physician professional liability insurance isn’t writing or renewing business at any price in Georgia, sending many of its longtime policyholders and insurance brokers scrambling to find replacement coverage.
Physician-owned or sponsored insurers seem to be faring better. Rate announcements from doctor-owned carries range from no increase to 30% with some 40% to 50% increases in so-called problems states such as West Virginia. Insurance companies there were created by medical societies and governed by physicians mushroomed in the 1970s and 80s in response to capacity and affordability problems during prior hard markets. Collectively, these companies now provide professional liability coverage to nearly two-thirds of the physicians in the U.S. Based on year-end 2000 financial statements, this physician-controlled segment of the insurance industry has generally done better than the medical malpractice industry as a whole., which includes the large commercial stock companies.
Rapidly Deteriorating Market
In a recent study, a leading research analyst for the insurance industry, Conning & Company, warned that the financial condition of the medical malpractice insurance business is rapidly deteriorating with “no margin for negative surprises.” Looking at the industry as a whole, it estimates a huge deficiency in malpractice claims reserves to the tune of a staggering $1.7 billion. This degree of adverse development clearly doesn’t happen overnight. Conning suggests the problem began as early as 1993 when the severity of incidents, accelerating claims payments, and increasing defense costs started to climb. Of particular concern as the rising incidence of claims alleging failure to diagnose and medication-related errors. Ophthalmologists should not be quick to assume that such problems don’t apply to them. A number of OMIC’s largest settlements have related less to the science and practice of ophthalmology and more to general medical problems such as failing to diagnose cancer or failing to adequately track and follow up on urgent care. Clearly, failure to follow some of the most basic risk management principles of general medical practice could have a more crippling effect on overall ophthalmic loss experience nationwide than some of the newer refractive procedures such as LASIK, which to date show only low to moderate claims severity.
What’s causing this deterioration and why does it seem to be occurring so suddenly after years of declining malpractice rates and aggressive competition? For at least the past five years, many medical malpractice carriers have had a voracious appetite for market share. This has had the effect of driving prices down even though combined and operating ratios for the industry as a whole were locked in a steady upward creep. Fortunately, physician-owned/sponsored carriers such as OMIC have been able to maintain more stable and consistent ratios during this period.
Nevertheless, during these “soft market” conditions, the financial results of carriers were propped up by good investment returns, favorable reinsurance deals, and better-than-expected loss results from prior policy years, which allowed companies to reduce their reserves and increase surplus. In the background, however, malpractice claims severity continued to grow. Defense costs kept rising, reserve takedowns on older policy years began to dry up, investment returns started to shrink, but premiums on the most part remained the same. Malpractice carriers were still locked in a battle to gain a shrinking market share. Artificially depressed rates prevailed until the damn burst at year-end 2000.
Loss Ratios Worsen
Every spring, insurance carriers file detailed financial statements showing the results of their operations through December 31 of the previous year. As analyst look at these year-end 2000 statements, a collective picture of the industry began to emerge. Their suspicions during the past 24 months are being confirmed. The approaching “hard market” has finally arrived. Loss ratios, which measure a company’s loss experience in relation to its total book of business, jumped nearly 10 points in one year to approximately 100% for all doctor-owned carriers combined. Analysts expect the numbers to be worse when large independent carriers are included in the mix. (OMIC’s loss ratio in comparison increased only 2.5% to 79.9% at year-end 2000.)
Other ratios that analyst use to measure an insurer’s operating performance also worsened during the past year. The combined ratio, which measures a company’s overall underwriting profitability before investment returns, climbed to 125% for the provider-sponsored carriers and 134% for the entire industry. However, after factoring gains on investments, carriers still showed relatively acceptable (albeit increasing) operating ratios. An operating ratio of less than 100 indicates acceptable financial health for a carrier because it is still able to show a profit from its core business. The average operating ratio for all doctor-owned carriers combined was 95.6% at year-end 2000. (OMIC reported a combined ratio of 119.3% and a favorable operating ratio of 91%.)
More Ominous Signs for Some Carriers
Unfortunately, we are now starting to see a number of long-standing carriers having difficulty even measuring up to the average. Data from financial filings for year-end 2000 indicate that between one-quarter and one-third of provider-sponsored medical malpractice carriers may show an operating ratio of greater than 100% and could report negative operating cash flow. A handful of companies may even show operating ratios in excess of 120%. A number of large independent commercial carriers also are facing significant challenges. The health care unit of one of the leading national medical liability carriers reported a year-end 2000 combined ratio of almost 130% and a fourth quarter combined ratio of nearly 160%. A large hospital association carrier reported a combined ratio last year of 200% as well as receiving two rating downgrades by A.M. Best Company in as many years.
Even if an improving U.S. economy turns Wall Street bullish again, it’s clear that it will take more than higher returns on investments to reverse the deterioration of some segments of the medical malpractice insurance industry. Insurance carriers will have to invoke a number of tough and unpopular remedies to exorcise the demons of the hard market of 2001. Here’s what ophthalmologists and doctors in all specialties can expect:
- Higher malpractice premiums for the near future. The size of increases will vary by carrier and depend on an individual company’s financial performance and overall profitability. OMIC anticipates a more modest fluctuation in its rates compared to the industry because it has historically kept rates a level sufficient to support its ability to pay claims over the long term.
- Tougher underwriting and cancellations of policies that carriers deem unprofitable. Some carriers appear to be engaged in wholesale cancellation of policies based primarily on geography, claims history, and scope of practice. Some uninsured ophthalmologists have called OMIC with stories of physicians being dropped because they were doing refractive surgery or had just one claim. OMIC plans to continue underwriting physicians in the same prudent manner it has in the past, relying on its historical success of selecting insureds who ultimately contribute to loss results that are consistently better than the industry.
- Fewer discounts and lower dividends. Cash-strapped carriers may become less generous with premium discounts to raise much needed revenue. Last year, the surplus of all companies combined fell for the first time in recent history. Shrinking surplus and smaller or nonexistent reserve takedowns could mean lower dividend payouts in the future. OMIC’s surplus did not decrease last year and was maintained as the same conservative level as the previous year. OMIC also is continuing its cooperative ventures with a dozen state and subspecialty societies and provides a special 10% premium discount for participating in cosponsored risk management programs. As in the past, dividends will be determined each year based on annual performance results.
-
Claims costs will continue to increase unless controls are employed. Despite the cyclical nature of insurance markets and litigation, claims severity will continue to grow unabated unless doctors and carriers employ proven measures to stem the tide.
- Tort Reform — First and foremost, efforts to bolster state tort reform initiatives are critical to keeping claims indemnity under control. According to Jury Verdict Research, jury awards in malpractice cases jumped 7% in 1999, raising the median award to $800,000. During previous, “medical malpractice crises,” the most common factor associated with markedly improved loss experience was the existence of strong tort reform measures with an effective cap on noneconomic damages (pain and suffering).
- Risk Management — Despite the temptation to reduce costs by cutting back on operational expenses, such as risk management, carriers instead need to provide more resources for these activities. OMIC will continue to make its ophthalmic-specific risk management activities available to policyholders and members of the American Academy of Ophthalmology and anticipates extending these activities through the Internet and other means.
Why Some Carriers Can Withstand a Hard Market
Perhaps the silver lining in this hard market is that physicians and their professional liability carriers have been down this road before. What we’ve learned from previous hard markets is that such a condition is not so much a malpractice crisis as it is a cycle. Fortunately, cycles turn, but their duration can clearly be impacted by how quickly and how well we respond. Physicians who didn’t chase some of those irresistibly cheap rates in the past and stayed with a strong and reasonably priced insurer are now in a better position to ride out the hard market with their current carrier. Companies that previously engaged in predatory pricing tactics to gain market share may now have to play “catch-up” by significantly boosting rates to meet the future demands of rising claims. Others, such as OMIC and those physician-sponsored carriers that remained focused on their original mission and purpose, are likely to successfully ride out the current storm as well as provide opportunities to those doctors now forced to search for a new carrier that can better support their long-term insurance needs.
In the future, adverse market cycles might be broken if some carriers choose to learn from the past and resist the temptation to feed their egos and corporate appetite for market domination and growth at any cost.
Coverage Issues, Policy Issues // No Comments
By Geri Layne Craddock, CLU
Vice President at Seabury & Smith, Washington, DC
[Digest, Summer 2001]
Have you ever stopped to consider how you would maintain your income if you were to suffer a disabling accident or illness? Many people believe that Social Security would be enough to protect them if they could not work. In fact, Social Security contains a very narrow definition of disability under which many situations are not covered. Additionally, Social Security benefits often are considerably less than private or group insurance benefits.
Workers’ compensation insurance should not be confused with disability insurance: workers’ compensation covers only disabilities that occur on the job. Disability plans offered by employers vary considerably in coverage length and percentage of salary that is covered. Many employers do offer disability insurance, but often it’s short-term coverage-generally one to five years-and may be grossly inadequate for those who suffer a long-term disability, such as paralysis or back injury.
At first glance, the cost of disability insurance might seem high. But it is important to remember that this is basic and essential insurance protection for people who rely on their regular income. According to the U.S. Census Bureau, one in five Americans were disabled in 1997.1 The American Council of Life Insurers maintains that a 35-year-old is six times more likely to become disabled than to die before he or she reaches age 65.2 Clearly, disability insurance may be even more essential than life insurance.
If you are interested in securing disability insurance, shop around and compare plans. Remember to weigh the cost against the potential benefits you would receive if you were unable to work for two, five, or twenty years. Examine these key elements as you compare policies:
Definition of “total disability.” This could be the most critical feature of your policy. Under many policies, you must be unable to perform any job for which you are qualified. One way to protect the educational investment you’ve made in your career is with own occupation coverage. Own occupation policies pay benefits if you are unable to engage in your own occupation even if you are able to return to work at a lower paying job that is not in your field. Some policies even consider a recognized medical specialty, such as ophthalmology, to be your occupation.
Length of benefits. Ideally, you should look for long-term coverage that protects you until age 65 even if you have to opt for lower benefits to keep the premiums more affordable.
Amount of coverage. To ensure that you have incentive to return to work, most plans set limits on the percentage of income you can insure, usually 50% to 60% of your total gross annual earnings. If you have an employer-provided plan that provides only limited coverage, consider purchasing supplemental coverage from another source.
Waiting period. The elimination or waiting period is the amount of time you must be disabled before your benefits begin-the shorter the waiting period, the higher the premiums.
Taxation of benefits. Benefits may be tax-free if you pay the premiums out-of-pocket, so check with your tax advisor.
Residual benefits. After a serious disability, many people return to work on a part-time basis for part-time pay. Residual or partial benefits can allow you to receive a combination of income and disability benefits until you fully recover. Without this feature, your benefits would most likely stop as soon as you return to work.
Financial strength of the insurance company. Find out as much as you can about the insurer. High ratings from A.M. Best Company, Standard & Poor’s, Moody’s Investors Service, or Fitch Ratings are good indicators of financial strength. Each of these independent rating companies have web sites where you can access information about various insurance companies.
Portable coverage. Having your own policy outside of your employee benefits allows you to move from practice to practice without fear of losing your coverage. Association-sponsored insurance is an excellent resource for that reason.
A valuable benefit of your Academy membership is your access to its many sponsored insurance programs, which have been individually tailored to meet the specific needs of ophthalmologists.
If you would like an information kit sent to you on the Academy’s Group Disability Income Plan,3 including plan features, cost, eligibility, renewability, limitations, and exclusions, call Seabury & Smith, the Academy’s life and health insurance administrator, at (888) 424-2308.
Notes:
- Taken from www.census.gov.
- Taken from www.insure.com/health/longtermdisability.html.
- Underwritten by New York Life Insurance Company, 51 Madison Avenue, New York, NY 10010.
Browsing articles in "Coverage Issues"
Coverage Issues, Policy Issues // No Comments
Digest, Fall 2001
Clarifications and guidelines already have been published to further elucidate the HIPAA statutes, with more to follow as health care providers try to make sense of HIPAA in relation to state privacy laws and their current practices. Simply stated, the law provides that a health care provider may not use or disclose protected health care information (PHI) except as required or permitted. Health care providers will need to provide patients with new blanket consent and specific authorization forms for the use of patient PHI. Ophthalmologists and anyone they share PHI with (such as companies providing accounting, billing, accreditation, or legal services) must enter into business associate agreements stating they will take appropriate safeguards in the transmission and use of PHI. When PHI is disclosed, providers may be require to de-identify the PHI or make reasonable efforts to disclose only the minimum information necessary to accomplish the intended purpose.
Under the new regulations, patients will have many affirmative rights that providers must facilitate. For example, patients can request restrictions on the use of their PHI; inspect, copy, and amend their PHI; and request an accounting of disclosures that providers have made of their PHI. In addition, health care providers must comply with certain administrative requirements, including: documenting their policies and procedures; appointing a privacy official; implementing privacy training; and creating administrative, technical, and physical safeguards to protect PHI.
HHS Encourage Cooperation
While much of this seems daunting, the U.S. Health & Human Services Department is encouraging cooperation and assistance to help providers achieve compliance – unlike the witch-hunt tactics of anti-fraud forces. Further, when assessing a practice’s reasonable compliance, the government will take into consideration a provider’s size and type of activities related to PHI. Punitive enforcement, at this point, is not a priority. However, civil fines, lawsuits, criminal fines, and imprisonment are tools the government can imply if it chooses to aggressively ferret out non-compliers.
What does this mean to OMIC insureds? First, it is essential that ophthalmologists understand the law and how it applies to their practice. Second, they must discern what steps to take to be compliant come spring 2003. Third, practitioners need to implement a protocol and then follow through on prescribed compliance procedures. Fourth, ophthalmologists should ensure that, as part of their insurance package, they are covered in the event the government targets their practice for violation of HIPAA regulations.
In light of the quickly approaching April 2003 deadline, OMIC is taking measures to protect and educate insureds. Risk management seminars will cover HIPAA privacy rules, and in early 2002, OMIC will make a HIPAA compliance program planning tool available to help insureds further understand the law and implement protocols to protect patient health care information.
Coverage for HIPAA Proceedings
Also in 2002, OMIC is expanding its fraud and abuse insurance program to cover HIPAA proceedings. OMIC’s Fraud & Abuse/HIPAA Privacy insurance will cover legal expenses civil proceedings instituted against the insured by a government entity alleging violations of HIPAA privacy regulations. OMIC’sComprehensive Fraud & Abuse/HIPAA Privacy insurance will cover legal expenses for proceedings instituted by a government entity alleging HIPAA privacy violations and resulting in administrative fines and penalties.
This HIPAA privacy coverage is in addition to both policies’ coverage of billing errors proceedings instituted by a government entity, third party payor, or qui tam (whistleblower) plaintiff under the False Claims Act. OMIC will provide a Fraud & Abuse/HIPAA Privacy policy free to its professional liability insureds $25,000 limits ($50,000 effective Jan 1, 2011). Higher limits to $100,000 are available for additional premium. OMIC offers American Academy of Ophthalmology members who are not OMIC professional liability insureds the opportunity to purchase this legal expense coverage as well. OMIC insureds and other Academy members also are eligible to purchase the Comprehensive Fraud & Abuse/HIPAA Privacy policy with limits ranging from $250,000 to $1,000,000. A $1,000 deductible applies to both policies.
For further information regarding OMIC’s new Fraud & Abuse/HIPAA Privacy program, please call the Sales Department at (800) 562-6642, ext. 654.
Coverage Issues, Coverage Question, General Insurance 101, Policy Issues // No Comments
By Kimberly Wittchow, JD, OMIC Staff Attorney
Digest, Spring 2004
Press coverage of the industrywide rise in medical malpractice claims frequency and severity is abundant. This has many insureds questioning whether their current limits of liability are adequate for the increasingly litigious environment in which they practice. To help insureds assess their coverage limits and needs, this article will address what is meant by limits of liability, how to select limits, and how changing limits affects coverage if a claim arises.
Your limits of liability are the maximum dollar amounts of indemnity OMIC will pay on your behalf as a result of covered claims. Indemnity is the amount of damages awarded in a lawsuit or agreed to in a settlement between the parties. OMIC will pay your reasonable defense costs in addition to your liability limits.
All OMIC insureds have two separate limits: the per claim, or “medical incident,” limit and the aggregate limit. The per claim limit is the maximum amount of indemnity OMIC will pay per insured for all damages caused by any one medical incident, or by any series of related medical incidents involving any one patient, regardless of the number of injuries, claimants or litigants, or the number of claims (notices, demands, lawsuits) that result. The aggregate limit, on the other hand, is the maximum amount OMIC will pay per insured for all claims made and reported during the policy period.
How to Select Limits
There are several factors to consider when selecting limits of liability. The limits you require may vary with changes in your state’s malpractice liability climate, the procedures you perform, and the makeup of your practice. Therefore, you should continually assess your current needs and corresponding coverage.
First, review the claims statistics for ophthalmologists. For example, as of February 2004, OMIC’s average indemnity payment was $130,166 and its largest indemnity payment was $1.8 million.
Second, consider your state’s risk relativity. When OMIC looks at risk relativity, it compares the number of insureds, the number of total claims, and the average indemnity paid per claim in each state. Under this analysis, due to the fact that OMIC has a large number of insureds in these states, OMIC’s highest claims activity is currently in California, Texas, and Illinois. For selecting limits, however, a better way to look at risk relativity might be to compare the average rate of claims per insured per state. OMIC insureds in Louisiana and Michigan currently experience the highest claims frequency.
Third, find out what liability limits your peers are carrying. The majority of OMIC insureds (65%) carry $1 million per claim/$3 million aggregate limits. Higher limits of $2 million per claim/and either $4 million or $6 million aggregate limits are selected by 21% of insureds. OMIC’s lowest offered limits of $500,000/$1.5 million are carried by 6% of insureds, while 4% select the highest limits OMIC offers, $5 million/$10 million. The remaining 4% of insureds carry other combinations of limits, including lower limits available exclusively to physicians who participate in their state’s patient compensation fund.
Fourth, consider the risks related specifically to your practice. Is your subspecialty one in which there is high claims frequency (e.g., cataract surgery) or large damage awards (e.g., neonatal care)? Do you share your coverage and limits with any ancillary employees or your sole shareholder corporation? On the other hand, have you ceased performing most surgical procedures or limited your practice to part time?
Fifth, assess your level of risk aversion. Would higher limits make you feel more secure because of the large indemnity cushion or less secure because of the “deep pockets” potentially discoverable by the plaintiff?
Finally, check with your hospital and state licensing board because they may specify the minimum amount of coverage you must carry. Also note that OMIC generally requires all OMIC-insured physiciansin practice together to carry the same liability limits. The practice’s legal entity cannot be insured at higher limits than those of the physicians.
Which Limits Apply to a Claim?
You should consider how changing your limits will affect the amount of indemnity available to you if a claim should arise. The limits of liability that apply to a claim are those limits that are in effect as of the date the claim is first made against you and first reported in writing to OMIC. In other words, if you increase or decrease your coverage after you’ve reported a claim made against you to OMIC, the limits that you carried when you reported the claim, not the new limits, will be applied to the claim.
Subject to underwriting review and approval, you may increase or decrease your limits of liability at any time during the policy period (although OMIC typically does not consider requests to change policy limits while a claim is pending). If you are in group practice, discuss this desired change with your practice administrator and partners. Your OMIC underwriter can provide you with the most recent OMIC data to help you determine which limits are appropriate for you. However, OMIC representatives are not in a position to offer you advice. If you need further assistance, please consult your personal attorney.
Claims Handling, Coverage Issues, Policy Issues // No Comments
By Kimberly Wittchow, JD
OMIC Staff Attorney
Digest, Winter 2005
In order to properly investigate and defend a medical malpractice claim, the professional liability company and the insured must cooperate. The participation of the insured, who is the subject of the lawsuit and holds first-hand information about the incident, is crucial to his or her own defense. Without such cooperation and assistance, the insurer is severely handicapped and may even be precluded from advancing any defense.
While the litigation process nearly always progresses successfully, there are times when some insureds thwart the resolution of their claims by failing to cooperate. Insureds may believe they have done nothing wrong and therefore avoid any work to counter the plaintiffs’ allegations. Or, afraid of the consequences, they may keep vital information away from their defense attorney until late into the case development. They might not understand the importance of their presence at litigation proceedings (such as depositions, mediations, or arbitrations) and worry about taking time away from their practice. Some attempt to handle matters “on their own” by discussing the case with plaintiffs’ attorneys against the advice of defense counsel or making payments without their insurers’ consent. Others may not want to tarnish their record and thus refuse to participate in settlement talks even when there is strong evidence that the standard of care was breached.
Investigation and Defense
That is why many professional liability policies contain Cooperation Clauses that require insureds to assist in the defense of claims made against them. OMIC’s policy has such a clause and, broken down, it requires the insured’s assistance on three levels. First and foremost, the policy requires that insureds assist in resolving the claim brought by the patient by helping with the insurer’s investigation and defense of the claim at trial or through settlement, as appropriate. This includes producing medical records, spending time with defense counsel, coordinating the appearance of staff at depositions or at trial, and attending court proceedings.
Coordination of Payment
The second situation is related to the coordination of payment among various legally responsible parties or insurers. The insured is required to cooperate in enforcing a right of contribution (where the loss will be shared) or indemnity (where another party is responsible for the entire loss) against someone else liable for the claim. For example, an insured may give notice under his or her OMIC professional liability policy for an office premises claim that might also be covered under the insured’s business owners or general liability policy. In this case, OMIC would ask the insured to help coordinate the defense and resolution of this claim with the other insurer.
Unauthorized Payments
Finally, the insured is prohibited from making payments, incurring other expenses, or assuming any obligations except at the insured’s own cost and with OMIC’s permission. OMIC wants to participate in its insured’s defense and work with the insured to come to the best resolution possible for the insured and the injured party. If the insured does not allow OMIC to participate, OMIC cannot be responsible for expenses the insured incurs. One example of this situation is where an insured decides, without the advice of defense counsel, to hire a private detective to track a malingering patient. This can be problematic for the defense because the defendant may be compelled to provide the plaintiff with this information. If nothing was revealed through the investigation, this could undermine the insured’s defense. Another example is when an insured, believing it is in everyone’s best interest, makes an out-of-pocket payment to the patient after a lawsuit has been filed. Again, if the case proceeds, this early payment to the patient may jeopardize its defense.
Even with the notice of required cooperation provided in the policy, some insureds still may not comply. The risk for these insureds is that they may be prevented from recovering under their insurance policies for the particular claim or they may lose their coverage altogether.
Before the situation reaches this level, however, the OMIC Claims staff would work diligently to educate the insured regarding the importance of his or her participation and cooperation in the defense of the claim and discuss what specific action is needed from the insured to bring him or her into compliance.
OMIC understands the issues that may impede a physician’s cooperation with his or her insurer and has several ways to assist its insureds with the upset of a lawsuit. First, OMIC provides access to one-on-one personal counseling (under the direction of the defense counsel in order to preserve attorney-client privilege) to help insureds deal with the emotional impact of litigation. OMIC also offers litigation and deposition handbooks to help insureds better understand the process. Finally, OMIC’s policy pays insureds for reasonable expenses incurred at OMIC’s request in the investigation or defense of a claim and for earnings lost as a result of attendance at court hearings or trials (see policy provisions for details).
Articles, Coverage Issues, Practice Issues // No Comments
By Kimberly Wittchow, JD, OMIC Staff Attorney
Digest, Fall 2005
Over the past year, a task force of OMIC Board and staff members, John W. Shore, MD, Anne M. Menke, RN, PhD, and Betsy Kelley, has been examining and revising underwriting requirements and risk management guidelines for coverage of outpatient surgical facilities (OSFs) insured by OMIC. OMIC’s Board of Directors assigned the task force to study scope of practice issues, state laws governing OSFs, and national, state, and local practice standards that establish a standard of care for cases performed in facilities insured by OMIC.
Types of Outpatient Surgical Facilities
First, the task force reviewed the type of facilities that OMIC insures. It found that OMIC insures a wide variety of OSFs with varying goals, scopes of business, and types of surgical procedures and anesthesia provided, including in-office surgical suites, refractive laser centers, and ambulatory surgery centers (ASCs). The types of anesthesia used in facilities insured by OMIC range from topical ocular anesthesia to full general anesthesia with invasive monitoring in high-risk surgical patients.
Some facilities are office-based treatment rooms where major eyelid and facial procedures are performed. Some of these offices permit outside surgeons of different specialties to utilize the in-office surgical suites. These surgeons, many of whom are not insured by OMIC, may perform major facial surgery in an unlicensed and loosely structured practice environment. This increases the vicarious liability shared by owners of the facility who are insured by OMIC.
Other surgical facilities are refractive surgical and laser centers. Surgical services in these facilities are usually limited to those requiring only topical anesthesia. The procedures are short in duration and the patients are relatively healthy. Some, however, are free-standing, licensed ambulatory surgery centers (ASCs), where surgeons of almost every specialty provide surgical services to a full range of pediatric, teenage, adult, and geriatric patients.
Review Process
Then the task force studied all of OMIC’s claims, suits, and settlements involving OSFs. The task force analyzed nursing, anesthesia, pediatric, and surgical standards by national professional groups as well as state and federal laws, regulations, and directives. Information gathered was used to revise existing underwriting requirements and risk management guidelines for OMIC insured OSFs. In addition to being discussed by both the Underwriting and Risk Management Committees, the proposed changes were extensively reviewed by consultants and practicing ophthalmologists with the goal of providing meaningful, clinically relevant, and workable requirements that cover all types of OSFs insured by OMIC. An anesthesiologist was consulted to review the anesthesia, monitoring, and emergency response requirements.
New Requirements
As a result of its work, the task force produced a rewritten and reformatted “Outpatient Surgical Facility Application” (OSFA), which was adopted by the OMIC Board of Directors. All ambulatory surgery centers, laser surgery centers, and in-office surgical suites used by physicians other than the owners and their employees will be required to complete the new OSFA. The OSFA contains detailed information about OMIC’s underwriting requirements pertaining to patient selection, type of anesthesia/sedation, pre- and postoperative assessments and monitoring, and emergency response and equipment. These requirements will be implemented immediately for all new OSF applicants and effective upon renewal in 2006 for facilities currently insured by OMIC.
It is important that insureds abide by all underwriting and notification requirements specified in the OSFA, as failure to do so could result in uninsured risk or termination of coverage. Working with OMIC’s experienced underwriters should enable insureds to complete the application, understand its requirements to avoid any coverage problems, and obtain an extension for those facilities that need additional time to comply with the requirements. While OSFs that are licensed or accredited may already meet or exceed these requirements, we anticipate that some OSFs may need additional assistance to implement them. Most accredited OSFs will receive a 5% premium discount for meeting the accreditation standards. There are helpful resources listed at the end of the OSFA itself and OMIC’s risk manager is available for confidential consultations.
All OMIC-insured physicians help bear the cost of defending claims and paying indemnity. It is incumbent on the OMIC Board of Directors, therefore, to protect OMIC insureds as a whole by establishing requirements that it believes will best limit the company’s liability and by making certain that insureds abide by these requirements, while at the same time offering physicians the ability to practice in various settings.
Articles, Claims Handling, Coverage Issues, Policy Issues // No Comments
By Kimberly Wittchow, JD
OMIC Staff Attorney
Digest, Winter 2006
Stress and worries abound when a patient sues or claims malpractice. One concern of insureds is the effect such action will have on their insurance coverage. Although claims can and sometimes do have an impact on insurability, understanding how a claim is handled at OMIC may provide insureds with some peace of mind.
Each department at OMIC has a different responsibility when a claim arises. Risk Management encourages insureds to be proactive and contact the department when medical incidents or issues occur so the risk manager can help them appropriately respond to the incident and incorporate any necessary changes in their practices or procedures. The Claims Department, in cooperation with the insured, wants to resolve the claim or lawsuit as efficiently and cost effectively as possible. Underwriting, meanwhile, must make certain that OMIC insures good risks. Insureds may therefore get several seemingly conflicting messages from the company depending on the status of their claim. Rest assured, however, that there are checks and balances in OMIC’s operational protocols to balance these priorities. Most importantly, OMIC’s Board of Directors is made up of ophthalmologists who not only approve company processes but also conduct claims and underwriting reviews.
Physician Review Panel
OMIC employs a continuous underwriting process, monitoring the claims activity of all insureds not only in anticipation of policy renewal, but also during the course of the insured’s coverage. Whether an insured’s claim(s) will warrant further review by OMIC’s physician review panel depends upon the insured’s history of claims frequency the number of claims or suits) and severity (indemnity amounts) and on the specific circumstances surrounding the claim(s). This could include indications that an insured is performing experimental procedures outside of the ordinary and customary practice of ophthalmology or has provided substandard care, followed poor informed consent techniques, or failed to cooperate during the claims-handling process. OMIC’s reviewers consider the insured’s entire claims experience, including his or her experience with insurance carriers other than OMIC.
After consideration, the physician review panel may determine one of several outcomes, including any of the following:
• The panel may continue the insured’s coverage without any conditions placed on his or her policy.
• The panel might continue the policy coverage with conditions, such as endorsing the policy to exclude coverage for certain activities or reducing the policy limits.
• The panel could also conclude that the insured’s risk profile falls outside of OMIC’s conservative underwriting standards, and that OMIC, therefore, is no longer in a position to cover the insured beyond the expiration of the insured’s policy.
• Finally, the panel, in rare circumstances, might determine that the insured’s actions warrant mid-term cancellation if the reasons for the cancellation fall within the policy provisions. These include fraud relating to a claim made under the policy and a substantial increase in “hazard insured against,” such as claims frequency or severity or unacceptable practice patterns.
Insureds are provided the opportunity to appeal coverage and termination decisions to the full Underwriting Committee. OMIC would not generally apply a policy surcharge (higher premium) because of claims experience.
Reporting a Claim or Medical Incident
The policy requires that an insured report to the Claims Department any claim or medical incident that occurs during the policy period which may reasonably be expected to result in a claim. The reporting of such an incident triggers coverage with OMIC. Even if the insured doesn’t obtain an extended reporting period endorsement (tail coverage) when he or she leaves OMIC, OMIC will continue to insure him or her for all covered claims and incidents reported while the policy was in force. An incident that does not develop into a claim will have no effect on the insured’s premium and will not be included in claims history reports provided to hospitals or other third parties. Claims or incidents reported to OMIC’s Risk Management Department are kept confidential: they are not shared with the Underwriting or Claims Departments without an insured’s permission and are not considered reported to OMIC for coverage purposes.
Finally, any indemnity payment made by OMIC on behalf of an insured will result in the removal of the insured’s loss-free credit upon renewal and for two policy terms. Then, if no further claims payments are made on behalf of the insured, the insured will begin earning loss free credits again, beginning at 1% and increasing 1% annually to a maximum discount of 5%.
Browsing articles in "Coverage Issues"
Articles, Coverage Issues // No Comments
Digest, Winter, 1996
Most physicians have heard these words: “Doctor, isn’t there anything else you can do? Some new treatment you can try?” Patients often perceive “new” treatments as better or less problematic than existing treatments and pleas for something “new” are particularly understandable when patients are faced with a terminal condition or loss of vision. Physicians generally look for proven treatments that will give their patients the best results with the least side effects. However, because of the tremendous psychological leverage of offering something “new,” physicians might be tempted to try unproven treatments to keep up with market forces. A good example of how market forces lead to such decisions might go something like this: “Mrs. Jones, you have the privilege of being in the right place at the right time. I am excited to inform you that I use the latest technology. The old method of correcting myopia is out and my new technique is in. In fact, because of my commitment to stay on the cutting edge, the laser I will be using to reshape your cornea was designed by me with the help of an engineer. This laser will correct your myopia in minutes.”
What assurance does Mrs. Jones have that the laser developed by her ophthalmologist is safe? Is it prudent for physicians to develop their own equipment or drugs for use on their patients? Who or what regulates physicians who do so?
Regulation of Physicians’ Practices
Production, sale, and clinical research of new drugs and medical devices are subject to regulation by the Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS). Physicians and patients are protected to some degree when they use drugs or devices that have undergone the scrutiny of the FDA and received approval for marketing and sale. The known indications, hazards, and adverse effects of the approved device or drug are required to be included in the product labeling. The FDA can restrict the ability of a company to sell, manufacture, or import a drug or device and can impose a variety of other protective measures.
Ordinarily, physicians are not directly regulated in their use of drugs and devices in day-to-day practice but are expected to practice in a manner that is designed solely to insure the well-being of the patient. Unless a physician is himself marketing or selling a drug or device, or acting as an investigator in clinical research, the FDA generally does not oversee or interfere with a physician’s individual practice decisions.
It is a different matter when the physician is conducting clinical research. Research activity occurs when the clinician conducts a “systematic investigation” designed to develop or contribute to “generalizable knowledge” such as by testing a hypothesis, drawing conclusions, and developing a base of knowledge from the results, especially concerning safety or effectiveness of the product.
If a practicing physician departs from usual (standard) treatments for an individual patient, such as by using a self-made device, a modified device, or a marketed drug for an off-label use, such use does not in and of itself constitute “research.” However, the non-standard treatment might constitute a deviation from accepted standards of medical care. The standard of medical care, which is based upon what reasonable physicians in the same specialty would do at the same time under similar circumstances, is overseen by state and local medical boards, and indirectly, by malpractice lawsuits. Issues of deviation from standards of care are usually raised by patients and/or other practitioners filing a complaint with their state or local medical board or by patients bringing malpractice lawsuits. The medical board also may be informed of aberrant practice styles by federal agencies, such as the Health Care Financing Administration and Medicare Peer Review Organizations.
In addition, local hospitals and clinics oversee standards of medical practice through credentialing, peer review, and quality assurance procedures, which involve investigations of standards of care, identification of deficiencies, and establishment of minimum qualifications for privileges. This is why malpractice insurance companies request information relating to licensing sanctions, peer review proceedings, and denial or surrender of hospital privileges.
So Mrs. Jones is protected by community standards, state and local agencies and institutions, and to a lesser degree, by agencies of the federal government.
Physician Practice versus Investigational Use
A physician may manufacture his or her own equipment or devices solely for his own use in his practice, and may use approved products for non-approved (off-label) applications. In fact, off-label use of medication is quite common. Distinguishing off-label use of drugs or devices as part of a physician’s practice from “experimental” or “investigational” use may be difficult at times. If the new use is based on firm scientific rationale and sound medical evidence, and is not for the purpose of developing information about safety or efficacy (for example, to support a request for FDA approval of the new use or to support advertising of new uses for the product), the use generally will qualify as “the practice of medicine” rather than “investigational use.”
However, if the physician is gathering new information on multiple patients, particularly for publication purposes or to obtain approval for a new device or new use, it is probably considered research, and the physician must comply with the panoply of federal statutes and regulations governing all aspects of approval of new drugs and devices, including numerous requirements for the protection of human subjects in research. In most cases, the practitioner will be required to obtain approval from his or her local Institutional Review Board (IRB), a committee that operates under HHS and FDA regulations for the purpose of protecting the rights of research subjects. The FDA will not accept research data in support of a new drug or device application unless the research protocol and consent documents have been reviewed and approved by an IRB. Similarly, peer reviewed journals usually require documentation of IRB approval before research results will be accepted for publication.
The FDA does have the right to request tracking of marketed products and, although off-label uses in medical practice are not generally regulated, the FDA can disapprove an existing product, require additional warnings, and/or request a recall of a product if it is not happy with off-label uses of the product. Otherwise, regulation of a physician’s medical practice generally falls under the jurisdiction of the state and local agencies previously discussed.
Liability and Insurance Issues
If a physician develops a new device, such as a new ultrasound for phacoemulsification to use on patients in the office, he or she may well face additional liability exposure for problems resulting from the use of the device. Injuries caused by the use of non-approved devices and drugs generally fall within the scope of malpractice and general liability coverage, but a physician may be exposed to personal risk as well if the insurance policy does not cover the liability associated with such uses.
For this reason, it is a good idea to contact your professional liability carrier about potential liability exposure if any of your practice activities involve the use of a self-made instrument or off-label use of drugs or devices regardless of whether they have gone through IRB approval. OMIC, for example, has carefully considered the use of the excimer laser in photorefractive keratectomy (PRK) and laser assisted in-situ keratomileusis (LASIK) and has developed internal guidelines intended to help reduce the increased professional liability and exposure to claims associated with their use.
Off-label applications of drugs also increase the potential for liability, although many drugs are widely used this way. A good example is mitomycin-C (Mutamycin) for glaucoma surgery. Mitomycin-C is FDA approved “for the use of disseminated adenocarcinoma in conjunction with other approved chemotherapeutic agents.” There is no mention of using this drug for pterygia or glaucoma surgery, and there is a long list of side effects for this drug, including pulmonary toxicity that does not appear to be dose related. Nevertheless, glaucoma surgeons are using this medication with increasing frequency, and even a few general ophthalmologists are using it to prevent the recurrence of pterygia following excision.
OMIC has considered the use of mitomycin-C and other off-label drugs (such as cyclosporin drops) and has this general recommendation:
When using a new or old drug in an approved manner, proceed with a green light. If it is an existing drug used in a non-approved manner (off-label), first consider whether its use poses significantly increased risks to the patient. Second, consider whether its use can be expected to bring good results without a higher complication rate. If it presents no more risk to the patient than that of daily living, proceed with its use; for example, the use of aspirin for anticoagulation after a central retinal vein occlusion. If there is an increased risk to the patient, ask yourself if at least a reasonable number of physicians in your specialty are using the treatment; that is, have peer reviewed articles been published supporting the use of the new treatment and is the treatment being used by a reasonable number of other practitioners with the same level of training as you?
Ophthalmologists are considered medical “specialists” and in most cases are held to a “national” rather than “local” standard of care. The same is true of subspecialists in various areas of ophthalmology. An ophthalmologist may face increased liability for off-label uses if a reasonable number of similar specialists or subspecialists are not using the same new treatment. An ophthalmologist whose patients experience more problems with off-label use of a medication than they would with standard methods of treatment might well be subject to professional criticism, and thus be exposed to malpractice liability and potential licensure or credentialing actions.
Additional Precautions
When considering new techniques, new devices, or new uses for approved drugs, practitioners need to think about taking additional measures to protect themselves as well as their patients. If you select a treatment for an individual patient with the intent that it will enhance the patient’s well-being, and there is sound medical evidence supporting the treatment, you are likely to be on solid ground. In such cases, FDA regulation is generally not controlling, and you probably will not need IRB approval unless the treatment so departs from standard practices that it may be considered experimental or investigational, or it presents significantly increased risks for the patient, and/or you wish to use the information for study purposes.
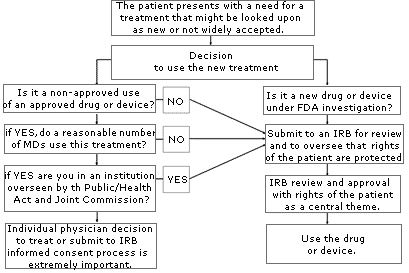
If you are uncertain about whether your use of a treatment or device might be considered “investigational” or “experimental,” consult your local IRB. An IRB is usually available at your local institution (some regional IRBs also exist) to review your proposed use and determine if it constitutes “research” requiring IRB approval. Similarly, if you are unsure whether the treatment has sufficient peer support or is simply too new, or if the literature is unclear, consider going through an IRB to help you with your decision. The flow diagram set forth on page A-78 may help you through the process of determining if you should submit your treatment proposal for IRB review.
Anytime you are considering unapproved uses of a device or drug, the informed consent process and its documentation, including treatment alternatives, should be thorough and specific in case you are later called upon to defend your decision to use non-standard treatments. If you have a specific area of concern, contact OMIC’s Risk Management Department for further information or referral to the appropriate resource or agency.
Coverage Issues, Policy Issues // No Comments
Anne M. Menke, RN, PhD, OMIC Risk Manager
Digest, Summer/Fall 2004
Allegations related to physician advertising are surfacing with increasing regularity in medical malpractice claims. In addition to alleging lack of informed consent, patients are using state consumer protection laws to claim that the physician defrauded them. This exposes the physician to punitive damages and other uninsured risks.
Physician advertising is regulated by state law as well as by the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) under provisions of the Food, Drug, and Cosmetic Act and the Federal Trade Commission Act (FTCA). The American Academy of Ophthalmology (AAO) and the American Society of Cataract and Refractive Surgery (ASCRS) have issued guidelines to advise their members on relevant ethical and professional standards.
Advertising “includes any oral or written communication to the public made by or on behalf of an ophthalmologist that is intended to directly or indirectly request or encourage the use of the ophthalmologist’s professional medical services … for reimbursement” (ASCRS Guidelines). These guidelines therefore apply to print, radio, and television advertisements as well as to informational brochures, seminars, videos, and the Internet.
The FTCA prohibits deceptive or unfair practices related to commerce and “prohibits the dissemination of any false advertisement to induce the purchase of any food, drug, or device.” The FTCA and the professional guidelines state unequivocally that advertising for medical and surgical services must be truthful and accurate. It cannot be deceptive or misleading because of (1) a failure to disclose materials facts, or (2) an inability to substantiate claims – for efficacy, safety, permanence, predictability, success, or lack of pain – made explicitly or implicitly by the advertisement. It must balance the promotion of the benefits with a disclosure of the risks and be consistent with material included in the informed consent discussion and documents.
Lack of Informed Consent Allegations
When not carefully crafted, advertising runs the risk of overstating the possible benefits of a procedure and potentially misleading patients into agreeing to undergo surgery without fully understanding or appreciating the consequences and alternatives.
In a sense, an advertisement becomes a ghost-like appendage to boiler-plate informed consent forms. If an advertisement overstates the benefits, misrepresents any facts, or conflicts with other consent documentation or patient education material, it can potentially make a jury believe the physician may have overstepped the line of ethical propriety by creating unrealistic patient expectations. Legally, such a scenario might allow a jury to conclude the patient was not given a full and fair disclosure of the information needed to make a truly informed decision.
Punitive Damages and Other Uninsured Risks
Another pitfall for the ophthalmologist who markets medical services are state laws that may allow the plaintiff to ask for punitive damages, which could double or treble the amount of money awarded to the patient by the jury. Physicians should be particularly concerned about such allegations since most professional liability insurance policies, including OMIC’s, do not pay for such damages.
OMIC’s underwriting guidelines state that advertisements and marketing materials must not be misleading, false, or deceptive and must not make statements that guarantee results or cause unrealistic expectations. In addition, insureds are required to abide by FDA- and FTC-mandated guidelines and state law. OMIC has specific policy language limiting its professional liability coverage to defense costs for claims related to misleading advertisements. No payment of indemnity will be made.
Therefore, if a plaintiff is alleging medical malpractice and has an added allegation of fraud, your OMIC policy will provide defense for both the allegation of malpractice and fraud but would limit any indemnity payment to awards related to the medical malpractice allegation of the lawsuit.
2021: Updated advertising medical services
Coverage Issues, Coverage Question // No Comments
By Kimberly Wittchow, JD
OMIC Insurance and Group Products Associates
[Digest, Fall 1998]
In the litigious business environment of the 1990s, ophthalmic practices of all sizes are increasingly vulnerable to employment practices liability suits. Small and large practices alike are discovering that discrimination, sexual harassment and wrongful termination suits can wreck havoc on their bottom line, as even frivolous or unsubstantiated claims must be defended, often at considerable expense to the practice.
The media is replete with stories of employers being sued even those who insist they “did everything right.” Take, for instance, the female employee who resigned and sued her former employer for sexual harassment in part because one of the company’s owners gave her a scarf on Valentine’s Day. The company claimed it investigated the situation after the employee complained and acted to protect her from further incidents. Nevertheless, a federal court jury awarded the plaintiff $82,000, which the company had to pay, along with its own substantial legal defense costs.
A case in the South arose when an accounting clerk in a medical office threatened to sue, stating that she was having sex with one of the directors. Previously a virgin, the woman claimed that on some occasions sex was consensual, but at other times she was forced to participate. Rather than face a Bible Belt jury, the practice opted to settle for over $35,000.
At one company, three former employees sued, alleging they were fired as a result of age discrimination. The three employees were able to prove that the rating system used by their employer to determine which employees to keep and which to terminate treated older employees unfairly. They won their case and were awarded $8.8 million in damages.
One young female doctor sued when she was not offered partnership after five years at a medical practice. Although she alleged that she had been discriminated against because of her gender and her pregnancy, the senior doctors claimed she was not offered partnership because her work was subpar. When new, potentially incriminating evidence came to light, the partnership settled for $22,500 and paid $5,000 in legal fees.
To make matters worse, damages in an employee suit for sexual harassment often do not end up with the employee’s claim against the offending co-worker. A recent trend has been a rapid rise in the number of counter complaints brought by the offending co-worker against the company for wrongful termination, demotion, defamation and breach of contract as a result of the harassment charges. These claims by supervisory level employees can be more expensive to defend and resolve than the underlying harassment suit.
Who Needs EPLI Coverage?
No ophthalmic practice is immune to lawsuits. Any practice with employees should seriously consider adding Employment Practices Liability Insurance (EPLI) coverage.
EPLI policies typically provide coverage for the health care entity; current and former appointed directors, trustees and officers; and current and former employees, including supervisory and managerial employees. Many carriers offer endorsements that allow other types of employees to be added to the policy. For instance, under the EPLI policy developed by OMIC and the American Academy of Ophthalmology, endorsements can be added to expand the definition of covered insured to include leased employees, independent contractors and leasing companies.
What Does EPLI Cover?
EPLI generally covers wrongful employment practices directed against any employee, former employee or employment applicant arising out of an employment relationship. OMIC offers an endorsement for third party coverage that includes claims brought by non-employees for sexual harassment or discrimination in the workplace. Wrongful employment practices covered by OMIC include:
- Discrimination on the basis of race, religion, age, sex, national origin, disability or any other protected class established pursuant to federal, state or local law.
- Harassment, sexual or other.
- Wrongful termination, including wrongful discipline or evaluation; retaliation; demotion; failure to hire or promote; or breach of employment contract.
In considering which EPLI policy to buy, it is important to evaluate additional coverage features. For example, OMIC’s ELPI policy automatically includes coverage for prior acts. This means that coverage is provided for claims made during the policy period arising from incidents unknown to the company that occurred before the policy effective date. If a practice does not require prior acts coverage, OMIC can exclude it from the policy and discount the premium accordingly. Another important feature of OMIC’s EPLI policy is a duty to defend clause. This requires the carrier to defend against all covered claims regardless of their legitimacy. OMIC includes the duty to defend as a standard coverage feature.
Your EPLI carrier should allow you to select from a broad range of coverage limits. For example, OMIC policy limits range from $50,000 to $2 million per claim and in the aggregate, including defense expenses. Various deductibles are available starting at $5,000.
Risk Management in the Workplace
Two recent U.S. Supreme Court rulings (Ellerth V. Burlington Industries, 118 S. Ct. 2257 [1998] and Faragher v. City of Boca Raton, 118 s. Ct. 2275 [1998]) reminded employers that they can defend themselves against sexual harassment claims when there are adequate and workable procedures in place that invite employees to complain and allow the employer to take appropriate action against the offender.
OMIC, likewise, realizes that prevention is the key to both a discrimination-and-harassment-free work environment and to cost containment in the employment practices litigation arena. In December, OMIC conducted its first nationwide risk management audioconference on employment practices liability. A one hour EPLI program was held at the OMIC booth in New Orleans in conjunction with the Academy’s annual meeting. Additional EPLI-related programs will be held in 1999.
For information on OMIC’s program, please contact the Underwriting Department at (800) 562-6642, extension 639.
Coverage Issues, Coverage Question, Policy Issues // No Comments
By Kimberly Wittchow, JD OMIC Staff Attorney
Digest, Spring 2005
It is important to OMIC that our insureds remain adequately protected from liability, especially during times of change and transition. By accepting your OMIC policy, you agree that the statements you made in the application are true. Most insureds supply accurate and thorough information both on the written application and in follow-up correspondence with their underwriter. However, after the underwriting process is complete and the insured is accepted for professional liability coverage, insurance may not be at the forefront of his or her mind. Nevertheless, it is important that insureds continue to communicate with their underwriter about any changes that occur in their practice.
Change in Practice Activities, Arrangements and Locations
The policy requires that insureds promptly inform OMIC, in writing, of any changes to the representations they made in their application. And insureds warrant, when signing the application, that they will update the information supplied on the application as necessary. This ensures that the coverage you have and the premium you pay correspond to your risk exposures.
• For instance, if you begin to perform a new refractive surgery procedure, you must complete and submit a supplemental questionnaire for this procedure. If OMIC grants your coverage request, your policy, which excludes coverage for all refractive surgery procedures, will be endorsed to provide coverage for the particular approved procedure.
• If you hire an optometrist or certified registered nurse anesthetist or bring in a locum tenens, you will want to ensure that he or she has his or her own insurance, or else is accepted for coverage by OMIC under your policy.
• You must alert OMIC when your practice arrangement changes, for example, if you join another practice, form a partnership with others, incorporate your practice, or allow outside utilizers to use your surgery facilities. You and your underwriter can discuss what options you have for deleting, adding, or otherwise changing your entity coverage and what limits are available or required for you to maintain.
• You must notify your underwriter if you significantly reduce your hours, eliminate certain surgical activities, or discontinue surgery altogether, so that your coverage can be amended and premium discounts, if any, can be applied.
• You must notify OMIC if you move or begin to practice in additional counties or states because your premium may need to be adjusted.
Claims, Complaints and Medical Conditions
While you already know to contact OMIC to report claims covered under your OMIC policy, you also must advise us of any claims filed against you that are covered by another carrier. Additionally, you must notify OMIC if a professional conduct complaint is filed against you, and as soon as you become aware of any proceedings or status changes regarding your license to practice medicine; your BNDD (drug) license, privileges at a hospital, HMO, or other medical facility; or your certification by or membership in a medical association, society, or board.
You are required to notify OMIC when certain life changes occur, for instance, if you have been treated for any medical condition that might impair your ability to practice, if you have been diagnosed with any mental illness, or if you have experienced any alcohol or drug dependency problems. If you are taking time off from your practice for maternity or paternity leave, you will want to alert OMIC because you may be eligible for a suspension in coverage.
Policy Endorsements and Coverage Reviews
While OMIC attempts to accommodate its insureds and their practice needs, changes requested by insureds are not always approved due to underwriting considerations. If requests are not made in a timely manner, there is no guarantee that we will retroactively amend your policy. Please remember that only endorsements or revised policy declarations, not simply notice, can waive or change the terms of your policy.
Under certain circumstances, changes that the insured notifies OMIC of may result in the insured being reviewed for continued insurability by OMIC. When certain increases in hazard are apparent or when membership criteria are not met (for example, if an insured loses his or her license or is no longer a member of the American Academy of Ophthalmology), OMIC may discontinue providing coverage to the insured.
The consequences of not notifying OMIC of changes will vary depending on the nature of the omission and the circumstances under which the omission is noted. These range from simply updating the information in your OMIC underwriting file to possible denial of a claim, or, in extreme cases, cancellation of your policy. Our goal is to encourage our insureds to notify us of changes to information we have about them in a complete and timely fashion to avoid any gaps in coverage.
Articles, Coverage Issues, Coverage Question, Policy Issues // No Comments
By Kimberly Wittchow, JD
OMIC Staff Attorney
Digest, Summer 2005
Whether you are new to a group practice or leaving a group to work for yourself or with others, you should be aware of how an OMIC group policy works and what to do if your practice situation changes.
A group that has OMIC professional liability insurance is usually issued one policy. The Declarations Page, which accompanies the policy, lists the ophthalmologists, CRNAs, optometrists, and any entities that are covered under the group’s policy. Under INSURED AND MAILING ADDRESS on the Declarations Page, the group name, or “policyholder,” is listed. This policyholder controls the group policy and is the main party with whom OMIC communicates about the policy.
Notice
Communications are often handled on behalf of the insureds of a group by the policyholder’s administrator or representative. OMIC assumes that, as a member of the group, the insured has given this representative the right to speak on the insured’s behalf regarding routine underwriting issues. While the administrator may initiate or facilitate a change in coverage, OMIC will seek the insured’s consent before changing the insured’s coverage limits, provisions, or classifications. Whenever possible, OMIC will communicate directly with an insured regarding any sensitive issues, such as licensure actions, substance abuse problems, or medical or psychiatric treatment.
Payment of Premium
Often, the business entity for the group will pay for each of the insured’s premiums under the policy. Nevertheless, each insured under the policy is considered by OMIC to “own” his or her own coverage. (Note, however, that for slot coverage for residents and fellows, the slot position, and not the individual in the slot, is the insured, and therefore the coverage is controlled entirely by the group practice.) This means that the insured is ultimately responsible for payment of his or her coverage under the policy. However, any refund of premium is credited to the policyholder, and it is the policyholder’s responsibility to distribute any refunds to individual insureds as appropriate.
Cancellation and Nonrenewal
Regarding cancellation and nonrenewal, the policyholder may request that OMIC delete an insured from a group policy. OMIC will try to get confirmation from the insured that he or she agrees with this termination of coverage. If OMIC cannot contact the insured, however, OMIC will process the termination, but will continue to attempt to communicate with the insured in order to determine whether he or she would like to remain insured with OMIC under an individual or another group policy.
Prior Acts Coverage
When joining a group, insureds may choose to purchase coverage for claims based on events which occurred before their coverage inception date under the group policy. Some groups do not allow the insured to acquire prior acts coverage under the group’s OMIC policy, while others may permit or require it.
Each insured under the group policy will have his or her own retroactive date which will reflect whether that insured has prior acts coverage. Some insureds will not need prior acts coverage because they are either new to practice or their prior acts are covered under another policy. This occurs when insureds were previously covered under an occurrence policy or bought an extended reporting period (tail) endorsement from their previous carrier. Remember that an insured’s retroactive date is usually not the same as the group entity’s retroactive date, and that the insured’s inception date may also be different from the group’s if the insured joined after the beginning of the group’s policy period.
Tail Coverage
Some groups require physicians to sign contracts when they join the group. Under these contracts, the group might require that, when a physician leaves the practice, he or she maintain coverage for the activities he or she participated in as a member of the group. This might take the form of purchasing a tail upon leaving the group, or proving that he or she maintains prior acts coverage under his or her new insurance policy after being deleted from the group policy. OMIC sends a tail offer directly to the insured upon termination of coverage. While it is ultimately the insured’s responsibility to obtain tail coverage if desired, a group may agree to pay for it. If the insured instead purchases prior acts coverage from his or her new carrier, the group might require that certificates of insurance be sent to the group periodically to ensure that the physician who left is maintaining his or her coverage for acts undertaken while with the group.
Browsing articles in "Coverage Issues"
Articles, Coverage Issues // No Comments
For OMIC’s current refractive surgery requirements (2022), please click here.
Coverage Issues, Policy Issues // No Comments
Examining premature infants for retinopathy of prematurity (ROP) is an important aspect of ophthalmic care. Ophthalmologists who perform this critical consultative function are providing a tremendous service to these infants and to the neonatal intensive care units (NICU) and supporting institutions that care for them. Because these institutions and ophthalmologists work together to reduce the likelihood that significant ROP will develop, they also should share the medical malpractice liability risk should a case of ROP advance to vision loss or blindness. If you perform ROP screening, you should know how your hospital handles this shared risk and take steps to limit your liability in the NICU.
Hold Harmless/Indemnification
One approach is to ask the hospital to hold you harmless and indemnify you for any liability you incur in performing ROP screening in the NICU. This means the hospital promises to absolve you of any responsibility for damages or other liability and to reimburse you for any loss you suffer arising from your provision of services in the NICU. This would be accomplished by inserting a hold harmless/indemnification clause in your ROP service contract with the hospital. Note, however, that many states limit the types of risks that can be transferred from one party (you) to another party (the hospital). Any indemnification agreement that you and the hospital enter into should be reviewed and/or drafted by legal counsel. Contact OMIC’s Legal/Risk Management Department for sample language.
An additional safeguard is for you to be named an “Additional Insured” under the hospital’s liability policy. This gives you direct access under the hospital’s policy to defense coverage for insured claims whether or not the hold harmless/indemnification provision is legally enforceable. However, “Additional Insured” status should not be obtained in lieu of a hold harmless/indemnification provision because the hospital’s insurance policy may not cover the loss.
Hospital-Provided or Funded Insurance
Another approach is for the hospital to provide you with additional insurance. Again, the specific provisions would be spelled out in your ROP service contract with the hospital. This hospital-provided insurance would coincide with your primary OMIC professional liability insurance. If you negotiate a primary or contributory policy with the hospital, then OMIC and the hospital most likely would share and cooperate in your defense and payment of any (covered) indemnity. (The OMIC policy describes how losses are apportioned when the OMIC policy and other insurance apply to the loss on the same basis.) However, if you negotiate an excess policy with the hospital, the hospital would not generally participate in the defense of the claim unless it is likely you will exceed your primary limits with OMIC. The excess limits would be available, though, if a judgment against you exceeds your policy limits with OMIC. Keep in mind that all determinations of coverage are case specific.
Another alternative is for the hospital to contribute toward payment of your insurance premiums. The AMA reports that hospitals are increasingly helping physicians pay their medical malpractice premiums to ensure that physicians continue to provide services at hospital facilities.
As an OMIC insured, one option for you is to raise your professional lia- bility limits and ask the hospital to reimburse you for the difference in premium. You should seek legal counsel when entering into these arrangements to ensure compliance with federal and state laws regulating hospital payments to physicians.
Damage Caps and Punitive Damages
When considering any of these options, you should be aware of state laws, such as those governing damage caps and the availability of punitive damages awards, because they will affect how much and what type of liability coverage you should seek. For example, if the state’s damage cap is $1 million and you have $2 million per occurrence/$4 million in the aggregate coverage, you can feel more secure that your limits will not be exceeded because of a jury award against you. However, if your state allows punitive damages awards, you might want to negotiate additional insurance from or indemnification by the hospital since the OMIC policy does not cover punitive damages. Your attorney should recommend the most appropriate and viable coverage alternatives and work with the hospital to draft the applicable terms.
You also should note that if a patient files a lawsuit, conflicts of interest may arise between you, the hospital, and other codefendants such as subsequent treating physicians. For example, you might disagree as to whose responsibility it was to provide follow-up ROP exams to a baby you examined once who was then transferred to another facility. In this situation, OMIC might exercise the right to separate counsel for its insured while still focusing on a unified defense.
Coverage Issues // No Comments
In February 2008, the FDA issued the following (excerpted) statement regarding Lipo-Dissolve:
“the FDA is aware of the practice of using Lipo-Dissolve. Lipo-Dissolve is not FDA approved for any use… there are no FDA- approved drugs with an indication to dissolve fat. FDA cannot assure the safety and efficacy of these types of drugs. These are unapproved drugs for unapproved uses and FDA cannot guarantee consumers’ safety… the use of compounded drugs is not considered “off- label” use… FDA approval of a drug includes approved labeling for use, and means that the FDA has evaluated the safety and efficacy of a drug for a specific use and population. Once approved, a drug may be prescribed by a licensed physician for a use that, based on the physician’s professional opinion, is appropriate…but it is expected that the physician is well-informed about the product and that the “off-label” use is based on sound scientific rationale and adequate medical advice…” numerous medical associations, including the American society of Ophthalmic Plastic and Reconstructive surgery, have issued warnings regarding injection lipolysis and cautioned their membership against performing such treatments. in addition, several states seek to ban or regulate Lipo-Dissolve procedures.
As a result of these developments, the Board of Directors has determined that OMIC will no longer extend coverage for any Lipo-Dissolve, mesotherapy, or similar procedure unless performed as part of an investigational drug trial under an American IRB-approved protocol.
Coverage Issues // No Comments
No area of ophthalmology is more controversial and difficult to underwrite than oculoplastic and oculofacial procedures. For this reason, OMIC has always had an oculoplastic specialist involved in making coverage decisions on what I will refer to here as “cosmetic” procedures. This includes past underwriting Committee chair, Michael J. Hawes, MD. Additionally, OMIC has maintained an ongoing educational cooperative venture with the American Society of Ophthalmic Plastic and Reconstructive Surgery since 1998. Thus, we feel very confident in our ability to assess the liability risks of ophthalmologists who perform cosmetic procedures and to establish the underwriting guidelines and requirements to minimize these risks. We believe it is because of these guidelines that we have a record of low frequency and severity of cosmetic surgery related claims.
Historically, these procedures were usually performed by oculoplastic specialists on established patients in a medical office or surgery center. With storefront medical spas now cropping up in malls and on street corners, patients can walk in and have laser hair removal, microdermabrasion, and other cosmetic procedures performed on the spot. Needless to say, this makes the underwriting process more challenging than when it simply involved individual ophthalmologists offering cosmetic procedures to their own patients in their own office or clinic.
Because cosmetic procedures are not generally included in ophthalmology residency programs, OMIC must verify that an applicant is qualified and has received the proper training to perform such procedures. Applicants are asked to provide information on the number of procedures they will perform annually, the areas of the body they will treat, the venue where they will provide these procedures, and their advertising of these procedures, if any. Applicants agree to abide by OMIC’s underwriting requirements and to inform us of any changes to their application responses. When OMIC is reasonably confident that the insured intends to offer these services in an ethical and professional manner with appropriate informed consent and preserve patient safety, coverage for cosmetic services will usually be approved.
The issue of where cosmetic procedures are rendered first arose in 2002 following FDA approval of cosmetic Botox. OMIC began to receive inquiries as to whether spas or even house parties were acceptable venues for injections. OMIC has been very clear that medical treatments such as Botox need to be provided in settings that have proper medical equipment and personnel.
“Medi-spas” present a hybrid environment that is not quite a medical office or clinic, but is more than a simple spa giving massages and facials. To further complicate matters, ophthalmologists may have an ownership interest in the medi-spa and/or serve as its medical director. Coverage for the liability risks associated with medi-spas may only be extended after an OMIC insured has completed a 10-page questionnaire. Failure to honestly complete this underwriting process puts the insured at risk of not being covered should a claim arise.
As long as ophthalmologists continue to expand their scope of practice, OMIC will continue to cover what they do and will work with insureds to carefully integrate new procedures into their practice with patient safety as the top priority.
Richard L. Abbott, MD OMIC Chairman of the Board

Browsing articles in "Coverage Issues"
Coverage Issues // No Comments
Digest, Fall 2012
The plethora of meningitis cases due to contaminated steroid injections has put compounding pharmacies under the microscope. Physicians are also being scrutinized for their part in prescribing and administering the tainted drugs. This article will look at ophthalmologists’ potential liability and the coverage OMIC’s policy provides should an OMIC insured be sued for prescription or use of contaminated compounded products.
Ophthalmologists use compounding pharmacies for a variety of products, including bevacizumab, Brilliant Blue-G (BBG), triamcinolone acetonide, and 5 percent Betadine. Compounded Trypan Blue, BBG, and Avastin have all been implicated in outbreaks of endophthalmitis. While no cases of ophthalmic injury from NECC products have been reported, its compounded betamethasone suspension was recalled due to potential contamination.
If a physician is named in a contaminated-product lawsuit, potential liability will depend on whether the plaintiff alleges product liability or professional liability (“medical malpractice”) and whether the court finds those claims applicable to the physician. Most state’s product liability laws provide for strict liability, which means a defendant can be held responsible without proof of fault. With strict product liability, all people or entities in the distribution chain are potentially liable. However, under some state’s laws, product liability claims against health care providers are not permitted. Product liability claims can also be based on negligence. A finding of negligence requires that the defendant breached a duty owed to the plaintiff, which caused the plaintiff to suffer damages.
There are three types of product liability defects: manufacturing, design, and failure to warn. A manufacturing defect occurs when the product is different than its design due to the manufacturing process. This includes contamination of the product during compounding as occurred at NECC.
A design defect means that the actual intended design of the product makes it unreasonably dangerous. In drug cases this usually means unreasonably severe side effects.
Failure to warn defects, also called marketing defects, occur when a product has improper or insufficient labeling, instructions, safety warnings, or recommendations for use. These marketing omissions can occur at the manufacturer, pharmacist, or provider level and often require a finding of negligence. The prescribing provider and even ancillary staff who instruct the patient on proper use of a drug or device may be liable as “learned intermediaries” between a drug’s manufacturer (or compounder) and the patient.
Medical malpractice, unlike product liability, always requires a finding of negligence. In this case, the breach of duty applies to the provision of medical services to the patient, not the sale of products. Therefore, in order for the court to determine whether product liability or malpractice should apply to a claim, it may attempt to determine if the provider “sold” the product. One way the court could do so is to look at the medical bills. Separate prices for the service (e.g. an injection) and the product (e.g. a steroid) could indicate a sale, whereas a global service charge or non-itemized bill would suggest a service.
If the prescription or administration of the contaminated product is considered a service, the plaintiff must show that the provider was negligent. For instance, did the provider fail to investigate the safety of the product being prescribed? Did the physician not obtain proper informed consent by failing to discuss all of the risks that went along with the use of the product? If the plaintiff can prove the physician breached this duty of care and the patient was harmed, the physician will be liable.
OMIC does not exclude coverage for an ophthalmologist’s prescription or use of compounded drugs or devices. OMIC respects the provider’s prerogative to select the most appropriate drug or device for a particular procedure or treatment for an individual patient even if it is off-label, unapproved, or compounded. OMIC’s policy covers insureds for allegations of medical malpractice based on an ophthalmologist’s direct patient treatment. This includes the prescribing or dispensing of medical supplies, devices, and drugs, including compounded products. However, OMIC’s policy does not cover product liability claims; it expressly excludes claims based on the designing, producing, manufacturing, assembling, distributing, marketing, or selling of any medical device or other product, including the failure to provide warnings or instructions with the product. If ophthalmologists will be reselling or otherwise participating in the distribution of products beyond direct patient treatment, they should secure separate coverage for product liability on a stand-alone basis or as part of a general liability package.
Sources:
American Academy of Ophthalmology, “Deadly Meningitis Outbreak Prompts Lawmakers to Consider Tighter Regulations on Compounding Pharmacies,” Member Alert, November 19, 2012.
Nick Brown, “Meningitis Lawsuits: Product Liability or Medical Malpractice?” The Insurance Journal, October 24, 2012.
Han W. Choi & Jae Hong Lee, “Pharmaceutical Product Liability,” Chapter 55, http://mofo.com.
Sarah Sellers and Wulf H. Utian, “Pharmacy Compounding Primer for Physicians: Prescriber Beware,” Drugs 2012: 72 (16): 2043-2050.
“Compounding Pharmacies – What Every Retina Specialist Needs to Know,” American Society of Retina Specialists Website.
“Overview of Medical Product and Drug Product Liability Cases,” Attorneys.com, http://www.attorneys.com/products-liability/overview-of-medical-product-and-drug-product-liability-cases/ (accessed November 8, 2012).
“Pharmaceutical Drug Liability,” Findlaw, http://injury.findlaw.com/product-liability/pharmaceutical-drug-liability.html (accessed November 8, 2012).
“Product Liability Claims Involving Pharmaceutical Drugs,” Nolo Law For All, http://www.nolo.com/legal-encyclopedia/product-liability-claims-pharmaceutical-drugs-30314.html (accessed November 8, 2012).
“Products Liability and Prescription Drugs,” Louisiana Insurance Litigation Blog, Thornhill Law Firm, October 31, 2008, http://www.louisianainsurancelitigation.com/2008/10/products_liability_and_prescri.html.
Coverage Issues, Policy Issues, Practice Issues, Surgery Issues // No Comments
Kimberly Wynkoop, OMIC Legal Counsel
Digest, Summer 2012
Sedation or anesthesia for ophthalmic procedures may be administered by anesthesiologists or other qualified anesthesia providers. Ophthalmologists are exposed to legal liability for claims based on the actions of anesthetists, and OMIC’s policy is available to protect ophthalmologists if they do arise.
CRNAs as Employees or Agents
Supervising ophthalmologists may be held vicariously liable for the acts or omissions of the CRNA under various theories of liability. The most common is respondeat superior, Latin for “let the superior respond” or “let the master answer.” Also termed the “master-servant rule,” this doctrine holds an employer or principal liable for the employee’s or agent’s wrongful (or negligent) acts committed within the scope of the employment or agency.
The fact that ophthalmologists are required to supervise nurse anesthetists’ provision of services during a procedure does not, by itself, create an employer-employee relationship, nor does it prevent ophthalmologists from maintaining independent contractor relationships with them (or no formal relationships at all, such as in a hospital setting). The substance of the relationship, not the label, governs the nurse anesthetist’s status as an employee or independent contractor. In order to determine whether a CRNA would be considered an employee, there are several factors to consider.
Does the ophthalmologist have a right to direct and control how the nurse anesthetist does the task for which he or she was hired? An employee is generally subject to the employer’s instructions about when, where, and how to work.
Does the CRNA bill separately for his or her own services? Independent contractors are more likely than employees to have non-reimbursed expenses and to bill separately for their own services. Whether under contract or not, an employee often will receive benefits and his or her compensation is subject to withholdings.
Control Over Independent CRNAs
As a general rule, ophthalmologists are not held liable for the negligent acts or omissions of independent CRNAs, even if—for billing and regulatory purposes—they are deemed to be their “supervisors,” unless the ophthalmologist controls or directs the actions of the anesthesia provider. Courts generally focus on the amount of control the treating physician exercises over the anesthesia provider to determine whether the physician should be liable for the anesthetist’s actions.
To determine if a physician has such control, courts consider who hired, could terminate, and pays the anesthetist, and who has the right to direct the anesthetist in the manner and performance of his or her work. The particular test to determine whether the supervising physician controls the anesthetist’s work varies by state.
In ASC and hospital settings, ophthalmologists are often required, under CMS regulations and/or state law, to supervise nurse anesthetists and sign various anesthesia-related orders, evaluations, and reports. It is OMIC’s understanding that the role of the treating physician, with relation to the provision of anesthesia services, is to (1) determine whether a patient requires the surgery or diagnostic procedure, (2) request that anesthesia be administered, and (3) determine that the patient is an appropriate candidate for the procedure and anesthesia. Therefore, it is not uncommon for the treating physician to be asked to sign perioperative orders for anesthesia, sedation, and anxiolytic drugs and to co-sign the pre-anesthesia evaluation conducted by the nurse anesthetist in addition to signing the record of the operation prepared by the circulating nurse as well as the dictated operative report. The fact that ophthalmologists sign certain anesthesia orders, evaluations, or records could be used by a plaintiff’s attorney to attempt to prove control, but without further evidence, it would probably not be sufficient.
Even if ophthalmologists do not have general control over a CRNA, the “borrowed servant” theory of liability provides that physicians can be held liable if they “borrow” another’s employee and acquire a temporary right of control over the employee that was originally possessed by the lending employer.
Negligent Supervision and Hiring
The supervising ophthalmologist may also be held liable for the CRNA’s actions under the theories of negligent supervision and negligent hiring. Negligent supervision arises from the rationale that physicians conducting professional activities through other professionals such as CRNAs are subject to liability for any injuries caused if the physician is negligent or reckless in supervising such activity. Negligent hiring may be alleged if the ophthalmologist knew or failed to use reasonable care to discover that the CRNA was not competent, fit, licensed, or certified to perform the required duties.
OMIC’s professional liability policy covers ophthalmologists for professional services incidents arising from direct patient treatment provided by “any person acting under the supervision, direction, or control of the insured at the time of the professional services incident, so long as that person was acting within the scope of his or her licensure, training, and professional liability insurance coverage, if applicable.” In other words, OMIC’s policy covers insureds for their liability arising from the supervision of nurse anesthetists, subject to all policy terms, conditions, and exclusions.
Coverage Issues, Policy Issues // No Comments
By Betsy Kelley OMIC VP, Product Management
Digest, Spring 2011
As the lead article illustrates, professional entities face a number of liability exposures. They have direct liability arising from administrative services the entity provides to the practice to facilitate the delivery of health care services. Such functions may include credentialing or supervisory activities, development of practice protocols, and maintenance of the premises. Under the doctrine of vicarious liability, liability for an injury may be assigned to a party who did not cause the injury but who has a legal relationship to the person who did act negligently. For entities, vicarious liability arises from the acts, errors, and omissions (“actions”) of the owners, employees, and other health care providers who render services to the practice’s patients. Ophthalmologist– owners of the professional entity may be held vicariously liable for direct patient treatment provided by others as well. To protect insureds from these exposures, OMIC extends coverage under two separate insuring agreements.
Coverage C—Professional Entities
Under Coverage Agreement C: Professional Liability Coverage for Professional Entities, coverage is extended to the professional entity for its direct liability arising from direct patient treatment provided by the entity and for its vicarious liability arising from direct patient treatment provided by any person for whose actions it is legally responsible, so long as that person was acting within the scope of his or her licensure, training, and professional liability coverage, if applicable. Coverage also applies under Coverage Agreement C to any person or entity affiliated with the insured professional entity in his, her, or its capacity as a member, officer, director, partner, or shareholder of the entity (“member”). This includes not only vicarious liability coverage for claims arising from direct patient treatment provided by others for whose actions they are legally responsible, but also coverage for claims resulting from professional committee activities the member performs for the insured entity. Professional committee activities include formal accreditation, utilization review, credentialing, quality assurance, peer review, and similar board or committee services. Coverage Agreement C does not cover members for direct liability arising from their own direct patient treatment or vicarious liability for the actions of others arising outside of that member’s role as an entity owner. (Ophthalmologists named in the declarations are covered under Coverage Agreement A for these liabilities.)
Vicarious liability coverage provided under Coverage C is conditional. If the claim results from a professional services incident involving direct patient treatment provided by a health care provider not insured under the entity’s policy, the provider must maintain professional liability insurance with a carrier acceptable to OMIC during the term of his or her employment, contractual relationship with, or utilization of the facility of, the insured entity. In the event the provider failed to maintain insurance as required, OMIC will not defend the entity or its members or pay damages or other payments resulting from their vicarious liability for the actions of the uninsured provider. This is why we ask you to provide certificates of insurance for all non- OMIC associates at each renewal. OMIC will defend an insured against allegations of vicarious liability for the actions of others based on an apparent partnership between the insured and another health care provider or professional entity, but supplementary payments and damages are excluded from coverage. If you share office space with health care providers who are not owners, employees, or formal independent contractors of your practice, please contact an underwriting representative to request a “Guide to Apparent Partnership.”
Coverage E—Premises
Limited office premises liability is insured under Coverage Agreement E. The entity and its members are insured for claims resulting from injury to a patient or property damage to a patient’s personal, tangible property caused by a professional services incident resulting solely from premises maintenance performed by the insured or anyone for whom the insured is legally responsible. Premises maintenance refers to the insured’s ownership, maintenance, or use of the office premises in which the insured provides direct patient treatment. Premises liability coverage is subject to a maximum limit of $50,000 per claim/$150,000 annual aggregate. Office misadventures that result from negligent supervision or are otherwise related to direct patient treatment are considered professional liability cases and are not subject to this sublimit. Coverage Agreement E does not constitute and is not meant to replace commercial general liability coverage or other fire and property coverage for the insured’s office premises.
Please note that no coverage will extend to an entity, its non-physician employees, or its members in their capacity as members unless the entity is named as an insured on the policy declarations. If your entity is not listed on your declarations and you would like to obtain entity coverage, contact your underwriter at (800) 562-6642, ext. 639, for an application. Similarly, if you form or acquire a new entity, change the name of your entity, or make any other change in your entity affiliation, please notify OMIC as soon as possible to minimize the risk of uninsured liability.
Articles, Coverage Issues // No Comments
By Ryan Bucsi, Mr. Bucsi is a Senior Litigation Analyst with OMIC’s Claims Department.
Digest, Winter 2007
You would never attempt to represent yourself in a medical malpractice lawsuit and assume responsibility for taking all the necessary depositions, preparing your own trial exhibits, examining witnesses during trial, and convincing a jury that your care and treatment met the standard of care. You know that if you are faced with a malpractice complaint, your first course of action should be to call OMIC’s claims department so we can put you in touch with a defense attorney who will represent you throughout the course of litigation.
What you may not know is that OMIC is also here to defend you if you receive a letter of investigation from your state medical board regarding patient care you have rendered. Some insureds have failed to report these letters of investigation until after they have responded on their own. Unfortunately, when there is a significant delay in reporting the investigation to OMIC, the results can be as catastrophic as attempting to defend your own malpractice lawsuit.
For example, an OMIC insured received a letter of investigation from her state board requesting a complete copy of a patient’s chart. There was no request or requirement that the insured provide a written description or narrative of the patient’s care, just a request for the chart. Without contacting OMIC for advice, the insured not only sent the requested records to the medical board but also a detailed narrative outlining her treatment of the patient. The insured did not hear back from the medical board until a year later when she received a letter notifying her that the board had concluded its investigation and was bringing disciplinary charges against her.
In the year that passed between the request for the patient’s chart and the notification of disciplinary action, the state board had been busy retaining experts who testified that the insured’s care was indeed below the accepted standard. Based on this expert testimony, the board proposed the following disciplinary action against the insured: a fine in the thousands of dollars, reimbursement of the costs associated with the state board investigation, a letter of reprimand, community service, and continuing education.
It was at this point that the insured contacted OMIC for assistance. An attorney was assigned to represent her and experts were retained on her behalf. Unfortunately, the insured had put herself at a great disadvantage by directly responding to the medical board, and no facts that OMIC or defense counsel presented could persuade the board to reverse its decision or reduce the proposed penalties. In the process, the insured’s defense coverage limits for this investigation were exhausted. Had the insured contacted OMIC as soon as she received the initial letter of investigation, OMIC would have assigned legal counsel to assist her in writing a response, which could have improved her chances for a more favorable decision from the state board.
OMIC Policy Covers Defense of Medical Board Investigations
Most physicians are not properly trained to respond to medical board inquiries and investigations in a manner that benefits their position. The initial letter from a state medical board may seem like a harmless request for records or information on a patient; however, your initial response is vitally important and may determine whether the board proceeds with an investigation or dismisses the complaint. Significantly, medical board or licensure actions can result in suspension of your medical license, thus making these cases far more risky than a medical malpractice case.
Insureds should treat a notice of medical board investigation the same way they would treat a patient complaint letter or request for information from a plaintiff attorney and contact OMIC before responding. OMIC defense attorneys are experienced in dealing with medical board actions and oftentimes are familiar with the individuals in charge of the investigations. This type of firsthand experience is invaluable when preparing a response to a letter of investigation and may reduce the likelihood that the medical board will pursue the investigation further.
Coverage for state board investigations is included as a part of your OMIC policy: “OMIC shall defend any insured ophthalmologist…against any investigation, disciplinary proceeding, or action for review (hereinafter “investigation”) of the insured’s practice by any federal, state or local regulatory agency arising from a complaint or report by a patient to such an agency of an injury to that patient resulting from a professional services incident involving direct patient treatment provided by the insured. However, OMIC will have no liability for fines, sanctions, penalties, or other financial awards resulting from the investigation.”
Please note that OMIC provides defense coverage only and there is a limit to this coverage: “The most OMIC will pay per insured for the claim expenses for any one such investigation is $25,000. The most OMIC will pay per insured for claim expenses for all such investigations during the policy period or the extended reporting period will be $75,000.”
It has been OMIC’s experience that meeting or exceeding the $25,000 expense limit is rare. In OMIC’s history, only six cases have reached or exceeded the $25,000 coverage limit. In fact, in a review of 46 closed medical board cases, the average expense for these matters was roughly $5,000. The attorneys assigned by OMIC to handle these cases are aware of this limited defense coverage and have negotiated their hourly fees with OMIC accordingly. This gives OMIC insureds the best combination of experience and value as our attorneys will attempt to resolve the matter within policy limits, thus avoiding out-of-pocket defense expenses for the insured.
Patient Complaint Often Precedes Malpractice Claim
A patient complaint to the state medical board has all the attributes of a malpractice claim except that the patient is not demanding money from the insured. OMIC’s rationale for providing defense coverage for medical board investigations is that these cases are often precursors to impending legal actions. A patient who complains to an investigative entity is most likely unhappy with the insured’s care and might later decide to file a medical malpractice claim against the insured.
State medical boards have a duty and a right to investigate patient complaints. Even if the allegations seem frivolous and you do not personally have concerns about your care and treatment of the patient, it is still wise to refer the case to OMIC so an attorney can respond on your behalf. Any OMIC insured is susceptible to these types of complaints; however, the majority of cases historically come from a handful of states, notably Florida, Arizona, and Nevada. OMIC has also defended state board investigations in California, Colorado, Texas, Illinois, Massachusetts, Washington, and Virginia. Regardless of which state you practice in, if you receive a notice of a state board investigation, please contact OMIC immediately.
When OMIC is brought in to defend these investigations early on, it has an excellent history of resolving them without fines or penalties being levied against the insured. Of 46 closed cases involving medical board investigations, 39 were dismissed without any type of adverse outcome for the insured. In all but two of these 39 cases, OMIC had assigned legal counsel on behalf of the insured. In the two cases that went before the state board without legal representation, the insureds did not report the complaint to OMIC until after they had responded to the initial letter of investigation. In the seven cases with adverse outcomes, the insureds were fined anywhere from $1,000 to $10,000 in addition to the costs of the investigation. They also were required to perform hours of community service and undertake continuing medical education. The complaints in these seven cases pertained to wrong site surgery, wrong surgery performed, or incorrect implantation of intraocular lenses.
It is important to note that once disciplinary action has been taken by a state medical board, it reports the action to the federation of State Medical Boards and the National Practitioners Data Bank. Furthermore, the physician is required to report any such action to other states where he or she practices or has a medical license. OMIC recommends that insureds consult with their OMIC-appointed attorney regarding reporting requirements of state board actions.
In summary, the same type of caution that is applied to medical malpractice claims and lawsuits should be applied to state medical board investigations. Insureds should contact OMIC’s claims department as coverage for such occurrences exists within your OMIC policy. OMIC has experienced defense attorneys to assist insureds in responding to such inquiries. The goal of legal representation is to decrease the likelihood that an investigation will proceed past the initial stages and result in the levying of fines or disciplinary action against the insured.
State Medical Board Actions
As a matter of public policy, the practice of medicine is a privilege granted by the people of the state acting through their elected representatives. It is not a natural right of individuals. Therefore, each of the 50 states, the District of Columbia, and the U.S. territories has a medical practice act that defines the practice of medicine and delegates the authority to enforce the law to a state medical board. In most states, the board regulates both allopathic and osteopathic physicians; in others, separate boards exist. There are currently 70 state medical boards authorized to regulate physicians.
Some of the functions of a state medical board include licensing physicians, investigating complaints, disciplining those who violate the law, conducting physician evaluations, and facilitating rehabilitation of physicians where appropriate. State laws require that boards assure fairness and due process to any physician under investigation.
Although medical boards sometimes find it necessary to suspend or revoke a license to practice, regulators have found that many problems can be resolved with additional education or training in appropriate areas.
In other instances, it may be more appropriate to place a physician on probation or place restrictions on a physician’s license to practice. This compromise protects the public while maintaining a valuable community resource in the physician. Probation and restrictions on a medical license may be in place while a physician receives further training or rehabilitation.
If a state medical board determines that a violation has occurred, it may take any of the following actions:
Reprimand or Censure – Physician receives a public admonishment.
Administrative fine/Monetary Penalty – Physician must pay a civil penalty fee imposed by the board.
Restitution – Physician must reimburse a patient or entity for monies improperly earned.
Probation – Physician’s license is monitored for a period of time.
Limitation or Restriction – Physician’s license is restricted in some way (e.g., a physician may be prohibited from performing specific procedures or prescribing certain drugs).
Suspension – Physician may not practice for a period of time.
Summary Suspension – Physician’s license is suspended immediately based on evidence that the physician’s practice presents a threat to public health and safety.
Voluntary Surrender of License – Physician surrenders license to avoid further disciplinary action.
Denial – Physician is not granted a license to practice or license is not renewed.
Revocation – Physician’s license is terminated and physician can no longer practice medicine.
To find out more about your state medical board, go to the federation of State Medical Boards’ website at www.fsmb.org/index.html.
Coverage Issues, Policy Issues // No Comments
By Betsy Kelley, OMIC Underwriting Manager
[Digest, Spring 1999]
Qualified eye banks may now purchase professional liability coverage from OMIC for direct and vicarious liability arising out of services they render. There are close to 100 eye banks in the U.S. to which OMIC might offer coverage and which might benefit from OMIC’s extensive expertise in ophthalmic underwriting, claims handling and risk management.
OMIC began exploring the feasibility of insuring eye banks last year at the request of a large insured network and its affiliated eye bank. A major factor in being able to sign on networks and other large ophthalmic groups is an insurer’s ability to provide coverage for eye banks, surgery centers and other related provider organizations affiliated with the network or group. After assessing the coverage needs and potential liability exposures of eye banks, the Underwriting Committee and Board of Directors determined that eye bank coverage would be a reasonable expansion of OMIC’s services to the eye care profession.
Who Qualifies?
Unlike other carriers that treat eye banks like any other “medical entity,” OMIC has carefully tailored an application form and underwriting guidelines specific to the activities and exposures of eye banks. To qualify for coverage, an eye bank must be a member of the Eye Bank Association of America or the American Association of Tissue Banks. As members of these organizations, eye banks must adhere to the medical standards developed by these organizations. OMIC has adopted these and other guidelines intended to reduce the likelihood of potential claims and to aid in the defense of any resulting claims. Additionally, the medical director or at least one board member must be a member of the American Academy of Ophthalmology for the eye bank to be eligible for coverage.
What is Covered?
OMIC also has crafted an Eye Bank Amendatory Endorsement to modify the standard policy terms and address the special liability issues of eye banks. For example, the definition of “professional services” has been modified to specifically include activities that eye banks perform: procuring, processing, testing, storing and distributing donor ocular tissue. In addition, the definition of “injury” has been broadened to include the possible allegations of disfigurement or mutilation of a cadaver and wrongful removal of tissue. Claims against eye banks are not common, but when they occur, most generally revolve around allegations that the donor tissue was obtained without the permission of authorized next of kin.
Coverage applies to the eye bank and its non-physician staff for their direct liability arising out of services they render. In addition, the eye bank is insured for its vicarious liability arising from services rendered on its behalf. Vicarious liability coverage arising from services rendered by employed or volunteer physicians applies provided these physicians maintain professional liability coverage themselves.
Policy Limits
Premiums for eye banks are based on a per-donation rate and vary based on the limits of liability selected and the eye bank’s retroactive date. OMIC offers a variety of limits ranging from $500,000 per claim/$1,500,000 aggregate to $5,000,000 per claim/$10,000,000 aggregate. (Lower limits of liability are available for eye banks that participate in their state’s patient compensation fund.) Limits apply on an indemnity-only basis. Defense costs are paid in addition to the limits, and unlike the coverages offered by other carriers that write eye bank coverage, OMIC’s coverage is “first dollar.” No deductible applies.
In addition to professional liability coverage, qualified eye banks also are eligible to purchase other insurance products available through OMIC, including coverage for directors and officers, errors and omissions, employment practices, Medicare/Medicaid fraud and abuse, workers’ compensation and business owners liability.
For more information on OMIC’s professional liability coverage for eye banks or other OMIC insurance products, please contact Betsy Kelley at (800) 562-6642, extension 630 or bkelley@omic.com.





































