Translate this page into:
English
Original Article
90 (
5
); 581-589
doi:
10.25259/IJDVL_854_2022
pmid:
37609732
, Khaled El Mulla, Abeer Alshaer, Heba M. Ashraf1, Eman A. Omran1
Department of Dermatology, Alexandria Faculty of Medicine, ElAzarita, Alexandria, Egypt
1Department of Microbiology, High Institute of Public Health, Alexandria University, ElAzarita, Alexandria, Egypt
Corresponding author: Dr Marwa Elsaeed Eldeeb, Department of Dermatology, Alexandria Faculty of Medicine, Elazarita, Alexandria, Egypt. marwa.eldeeb16@alexmed.edu.eg
- Received: 2022-09-26, Accepted: 2023-03-08, Epub ahead of print: 2023-07-21, Published: 2024-08
© 2024 Indian Journal of Dermatology, Venereology and Leprology - Published by Scientific Scholar
How to cite this article: Eldeeb ME, El Mulla K,Alshaer A, Ashraf HM, Omran EA. The effect of long-pulsed 1064 nm Nd:YAG laser-assisted hair removal on some skin flora and pathogens: An in vivo study. Indian J Dermatol Venereol Leprol. 2024;90:581-9. doi: 10.25259/IJDVL_854_2022
Abstract
Background
The effect of NDYag on normal skin flora and pathogenic microbes has not been studied.
Objectives
Evaluation of immediate (before versus after each session) and delayed (pre-first session versus pre-fourth session) antimicrobial effect of Nd:YAG laser-assisted hair removal.
Methods
Thirty females scheduled for axillary Nd:YAG laser hair removal were included. Skin swabs were collected from the vault of the dominant axilla before and after each of the four sessions. Bacteriological cultures were performed to record the counts of total aerobes, total anaerobes, lipophilic bacteria, total staphylococci, Staphylococcus epidermidis (S. epidermidis), S. saprophyticus, S. hominis, and S. aureus. Reported changes in sweat odour and folliculitis (if present) were recorded.
Results
S.hominis was the predominant species in all subjects before and after all sessions. Counts of total aerobes, total anaerobes, lipophilic bacteria, total staphylococci, and S.hominis significantly decreased after all 4 sessions. A significant reduction was noted in the median colony counts before the fourth session as compared to the baseline count before the first session in total aerobes (278.9 versus 126.3 × 105 CFU/cm2, p = 0.003), total anaerobes (338.7 versus 103.7 × 105 CFU/cm2, p = 0.002) and total staphylococci (248.5 versus 105.0 × 105 CFU/cm2, p = 0.004). Most subjects reported worsened or unchanged axillary sweat odour. There was a statistically significant positive correlation between sweat odour and the counts of total aerobes (r = 0.433, p = 0.017), total anaerobes (r = 0.377, p = 0.040), total staphylococci (r = 0.383, p = 0.036) and S.hominis (r = 0.497, p = 0.005) ; lower counts were associated with a worsened odour.
Limitations
Small sample size; few laser sessions; short follow-up; subjective assessment of sweat odor and quantity.
Conclusions
Laser caused an immediate and delayed reduction in axillary aerobes, anaerobes, lipophilic bacteria, and staphylococci. This form of dysbiosis might lead to sweat odour changes.
Keywords
Nd:YAG laser
skin flora
staphylococci
folliculitis
total aerobic count
Show Related Articles from PubMed
Introduction
Laser hair removalunwanted hair.1
Absorption by melanin at 1064 nm is lower than at shorter wavelengths but is still sufficient to permit selective photothermolysis of the pigmented hair follicle with permanent destruction.3,4 This reduces the thermal damage to the surrounding epidermis, making Nd:YAG laser the safest option in dark-skinned patients.5
A distinctive axillary odour emanates when a large and permanent population of microorganisms thrives on secretions from eccrine, apocrine, and sebaceous glands. The resident axillary microbiota consists mainly of bacteria of the genera Staphylococcus, Micrococcus, Corynebacterium, and Propionibacterium. In females, Staphylococci predominate over Corynebacteria.6 The use of laser for hair removal can alter the microbial flora, and an alteration of the smell of sweat can accompany this. The duration of this antimicrobial effect is undetermined, and so are the accompanying skin changes such as sweat odour and amount.7
Studies on the effect of Nd:YAG laser on skin flora are scarce. The present study aimed to evaluate the effect of Nd:YAG laser hair removal on the density of some skin flora and pathogens in the axilla (both immediate and delayed effects), and the relation between changes in bacterial counts and changes in axillary sweat odour and amount.
Subjects and Methods
This quasi-experimental interrupted time series study (ClinicalTrials.gov ID NCT05034237) was conducted on 30 females recruited at the dermatology outpatient clinic of Alexandria Main University Hospital, Egypt over 10 months from January to November 2021. Approval of the Medical Ethics Committee of Alexandria Faculty of Medicine was obtained (IRB NO 00012098). Informed written consent was taken from every participant.
Participants aged 20–40 years were selected, who had Fitzpatrick skin types III–V, and had had no previous laser hair removal sessions involving the axillae. Those with inflammatory skin conditions such as atopic dermatitis or psoriasis, or fungal or herpetic infections were excluded, as well as those with diabetes, those receiving immunosuppressive therapy, and pregnant or lactating females. A pre-designed data sheet was used to collect patients’ data as their age, the number of laser session, their skin type, history of folliculitis as well as their laboratory results. Results of changes in sweat odour and amount were also recorded in the last session as compared to the first session.
Laser sessions
All participants were subjected to four sessions of laser-assisted hair removal at 4 week intervals using long-pulsed 1064 nm Nd:YAG laser (Cynosure Elite+™, Westford, USA). Parameters were set (15–18 mm spot size, fluence 24–35 J/cm2, pulse duration 30–40 ms) according to hair colour, thickness, and density, and the skin phototype. All participants were instructed to avoid using oral or topical antibiotics for 72 hours before sampling, avoid washing the axilla with soap for 24 hours before the session, avoid using deodorants, antifungal or antibacterial cleansing products in the axilla throughout the study, and not to use a topical anaesthetic before sessions.
Sample collection
Swab were taken immediately before and after the session, as any time lapse would have affected the bacterial count (bacteria multiply with time). Each time (pre- or post-laser), two sterile swabs were used simultaneously for skin swabbing; these were then placed in 2 ml sterile thioglycolate broth (an enrichment broth that supports the growth of anaerobes, aerobes, and microaerophilic microorganisms). A modified standardized swabbing method described by Selwyn and Ellis (1972) was used.8 The moistened with sterile saline swabs were vigorously rubbed on a 2 × 2 cm2 area of the right axilla, with moderate pressure for 30 seconds. The tube into which the two swabs were placed was labeled, placed in an ice box and transported within 2 hours to the Microbiology Laboratory at High Institute of Public Health, Alexandria University for immediate processing.
Laboratory methods
The tube containing the two swabs and thioglycolate broth was vortexed for 30 seconds to disperse any bacteria adhering to the swabs and to distribute bacteria evenly in the liquid broth. A volume of 1 mL of the broth was transferred to each of six dilution tubes to obtain dilutions from 10−1 to 10−6. These dilutions were used to culture the required plates. An automated pipette took a volume of 100 µl from each dilution tube to culture each of the following plates: two blood agar plates (for the total bacterial count; one aerobic and the other one for anaerobic incubation), mannitol salt agar plate (for enumeration of total Staphylococci and detection of S.aureus), blood agar supplemented with 0.5% Tween 80 (for enumeration of lipophilic bacteria) and Staphylococcus Chromogenic Agar plates (Condalab, Madrid,Spain, # Cat. 2076) for staphylococcal species identification and enumeration. All plates were incubated at 37°C for 24–48 hours aerobically, except for the blood agar plate reserved for the enumeration of anaerobes which was incubated using gas packs at 37°C for 5 days.
Bacterial colony counting and identification procedures
Plates from dilutions showing the best countable colonies (30–300 colony forming unit (CFU)/plate) were chosen to be counted. Identification of S. aureus on mannitol salt agar was done according to standard microbiological methods.8 Isolates growing on Chromogenic (CHROM) agar were identified based on their colour according to the manufacturer’s instructions,9 and biochemical tests were used to confirm the identity of the isolates. S.aureus was tested for cefoxitin susceptibility to identify methicillin-resistant S. aureus (MRSA).8 S.hominis were identified by being coagulase-negative, followed by automated identification by Vitek 2 Compact (bioMérieux, France).
The final colony count was calculated in relation to the swabbed surface area and dilution used, and the colony count was expressed as colony forming units (CFU)/cm2. Automated bacterial identification using Vitek 2 Compact (bioMérieux, France) was done when needed to confirm isolates.
Assessment of sweat odour and quantity and presence or absence of folliculitis
Participants were asked about changes in sweat amount and odour by the end of the study (fourth laser session). Change in the amount of sweating was classified as decreased, increased, or unchanged, while change in odour was expressed as a score: improved (+1), worsened (–1), or unchanged (0). Any history of recurrent axillary folliculitis was re-evaluated by the end of the study.
Statistical analysis
Data was fed to the computer and analysed using IBM SPSS software package version 25.0. Qualitative data were described using numbers and percentages. The Kolmogorov-Smirnov test was used to verify the normality of distribution, data were not normally distributed. Quantitative data were described using the range (minimum and maximum), mean, standard deviation, and median. Significance was judged at the 5% level. The tests used were: Wilcoxon signed-rank test as a non-parametric test used for quantitative variables to compare between two related groups (paired comparison), Friedman’s test as a non-parametric test used for quantitative variables to compare between more than two related groups (paired comparison), Spearman’s rho correlation (for abnormally distributed quantitative variables) and Mann-Whitney test for quantitative variables to compare between two independent groups.
Results
The mean age of our participants was 26 years, with skin phototypes III-V. Five subjects gave a history of recurrent folliculitis.
The immediate antimicrobial effect of Nd:YAG laser
This was evaluated by comparing bacterial counts before and after each laser session. With the first session, there were significant reductions (p < 0.001) of colony forming units (CFU/cm2) of total aerobes (278.9 versus 111.3 × 105), total anaerobes (338.7 versus 66.3 × 105), lipophilic bacteria (205.0 versus 78.7 × 105), total staphylococci (248.5 versus 85.0 × 105) and S. hominis (120.0 versus 25.0 × 105) [Table 1][Figure 1]. This pattern of reduction was also found in the second, third and fourth laser sessions.
| Type of bacteria | Colony count (×105 CFU/cm2) | p-value | |
|---|---|---|---|
| Before first session | After first session | ||
| Total aerobes | |||
| Mean ± SD | 778.3 ± 1429.7 | 511.4 ± 1318.7 | Wilcoxon Signed Rank test = 30.0 P < 0.001* |
| Median | 278.9 | 111.3 | |
| Min-Max | 2.5–6250.0 | 0.0–6012.0 | |
| Total anaerobes | |||
| Mean ± SD | 787.3 ± 1422.9 | 305.1 ± 693.4 | Wilcoxon Signed Rank test = 30.0 P < 0.001* |
| Median | 338.7 | 66.3 | |
| Min-Max | 2.5–6662.5 | 0.0–3737.0 | |
| Total staphylococci | |||
| Mean ± SD | 408.6 ± 536.1 | 455.9 ± 1703.6 | Wilcoxon Signed Rank test = 37.0 P < 0.001* |
| Median | 248.5 | 85.0 | |
| Min-Max | 12.5–2720.0 | 0.0–9425.0 | |
| Lipophilic bacteria | |||
| Mean ± SD | 936.5 ± 2268.7 | 244.7 ± 646.2 | Wilcoxon Signed Rank test = 19.0 P < 0.001* |
| Median | 205.0 | 78.7 | |
| Min-Max | 0.0–9262.0 | 0.0–3532.5 | |
| S.epidermidis | |||
| Mean ± SD | 1.4 ± 7.3 | 4.8 ± 22.0 | Wilcoxon Signed Rank test = 4.0 P = 0.593 |
| Median | 0.0 | 0.0 | |
| Min-Max | 0.0–40.0 | 0.0–120.0 | |
| S.saprophyticus | |||
| Mean ± SD | 9.4 ± 27.6 | 11.4 ± 53.3 | Wilcoxon Signed Rank test = 8.0 P = 0.161 |
| Median | 0.0 | 0.0 | |
| Min-Max | 0.0–125.0 | 0.0–292.0 | |
| S.hominis | |||
| Mean ± SD | 246.3 ± 300.6 | 108.4 ± 157.6 | Wilcoxon Signed Rank test = 25.0 P < 0.001* |
| Median | 120.0 | 25.0 | |
| Min-Max | 3.4–1283.7 | 0.0–578.5 | |
P*: p-value significant at level < 0.05
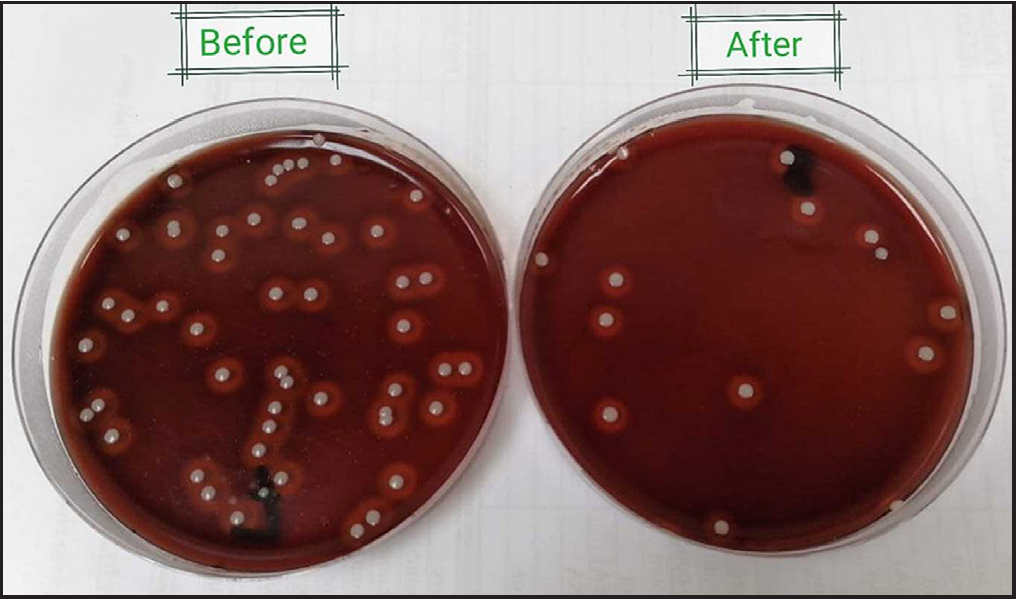

- Blood agar plates showing total lipophilic bacterial counts before (left) and after (right) the third session of one participant.
The delayed antimicrobial effect of Nd:YAG laser
This was evaluated by comparing bacterial counts before the first and fourth laser sessions. There was a significant reduction in the median colony counts (CFU/cm2) for total aerobes (278.9 versus 126.3 × 105, p = 0.003), total anaerobes (338.7 versus 103.7 × 105, p = 0.002) and total staphylococci (248.5 versus 105.0 × 105, p = 0.004). There were also observed reductions in the median lipophilic bacterial and S.hominis counts, but these did not reach statistical significance [Table 2].
| Type of bacteria | Colony count (×105 CFU/cm2) | p-value | |
|---|---|---|---|
| Before first session | Before fourth session | ||
| Total aerobes | |||
| Mean ± SD | 778.3 ± 1429.7 | 172.6 ± 176.6 | Wilcoxon Signed Rank test = 89.0 p = 0.003* |
| Median | 278.9 | 126.3 | |
| Min-Max | 2.5–6250.0 | 1.0–762.5 | |
| Total anaerobes | |||
| Mean ± SD | 787.3 ± 1422.9 | 400.6 ± 1069.7 | Wilcoxon Signed Rank test = 84.0 p = 0.002* |
| Median | 338.7 | 103.7 | |
| Min-Max | 2.5–6662.5 | 2.5–5037.5 | |
| Total staphylococci | |||
| Mean ± SD | 408.6 ± 536.1 | 510.1 ± 1659.0 | Wilcoxon Signed Rank test = 91.0 p = 0.004* |
| Median | 248.5 | 105.0 | |
| Min-Max | 12.5–2720.0 | 1.5–8612.5 | |
| Lipophilic bacteria | |||
| Mean ± SD | 936.5 ± 2268.7 | 189.3 ± 295.5 | Wilcoxon Signed Rank test = 144.5 p = 0.070 |
| Median | 205.0 | 110.0 | |
| Min-Max | 0.0–9262.0 | 0.0–1250.0 | |
| S. epidermedis | |||
| Mean ± SD | 1.4 ±7.3 | 4.0 ±21.9 | Wilcoxon Signed Rank test = 3.0 p = 1.000 |
| Median | 0.0 | 0.0 | |
| Min-Max | 0.0–40.0 | 0.0–120.0 | |
| S.saprophyticus | |||
| Mean ± SD | 9.4 ± 27.6 | 18.3 ± 47.7 | Wilcoxon Signed Rank test = 35.0 p = 0.445 |
| Median | 0.0 | 0.0 | |
| Min-Max | 0.0–125.0 | 0.0–237.5 | |
| S.hominis | |||
| Mean ± SD | 246.3 ± 300.6 | 145.8 ± 198.8 | Wilcoxon Signed Rank test = 150.0 p = 0.090 |
| Median | 120.0 | 70.0 | |
| Min-Max | 3.4–1283.7 | 0.0–825.0 | |
P*: p-value significant at level <0.05*
With regard to staphylococcal species, S.hominis represented 96.6% and 83.3% of species before the first and fourth sessions, respectively. S.saprophyticus comprised 3.3% and 10% before first and fourth sessions respectively, while the corresponding figures for S.epidermidis were 0% and 3.3% respectively (data not shown). Four participants yielded S.aureus transiently in their axillary swabs; 3 yielded S.aureus prior to their third laser sessions with a median colony count of 10 × 105 CFU/cm2) and one participant had positive S.aureus both before and after her third laser session with colony counts of 307.5 × 105 and 5 × 105 CFU/cm2 respectively. None of the S.aureus isolates were found to be MRSA, as they were all sensitive to cefoxitin.
On comparing the median bacterial counts before each session, there was a significant difference between the four sessions in total aerobe counts (post hoc test, p = 0.010), total anaerobe counts (post hoc test, p = 0.008), total staphylococci (post hoc test, p = 0.001), and S.hominis (post hoc test, p = 0.036). Comparing the median bacterial counts after each session revealed a significant difference between the four sessions in total aerobes (post hoc test, p = 0.002), total anaerobes (post hoc test, p < 0.001), total staphylococci (post hoc test, p = 0.031), and lipophilic bacteria (post hoc test, p = 0.022). [Figures 2 and 3].
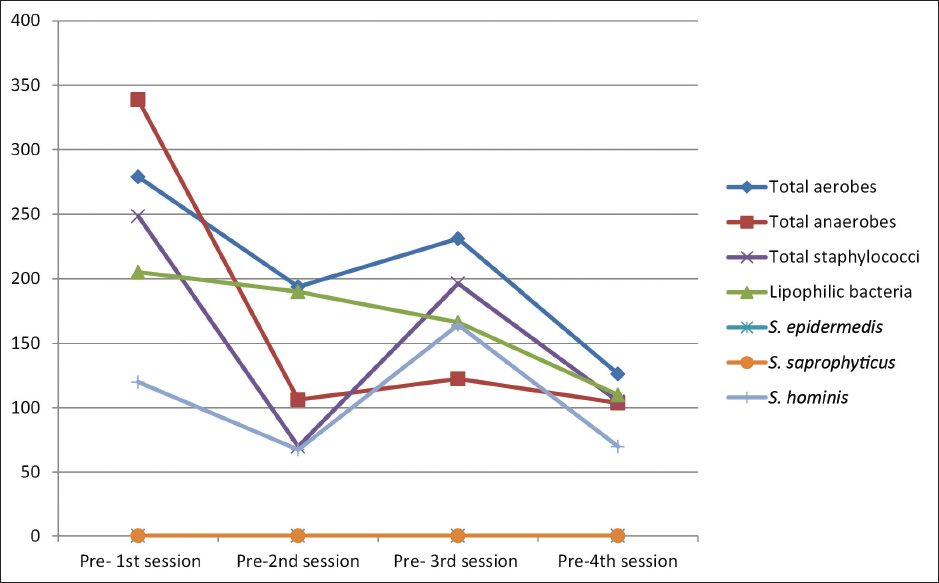

- Distribution of the studied participants (n = 30) according to the median bacterial colony count before each session (S1, S2, S3 and S4).
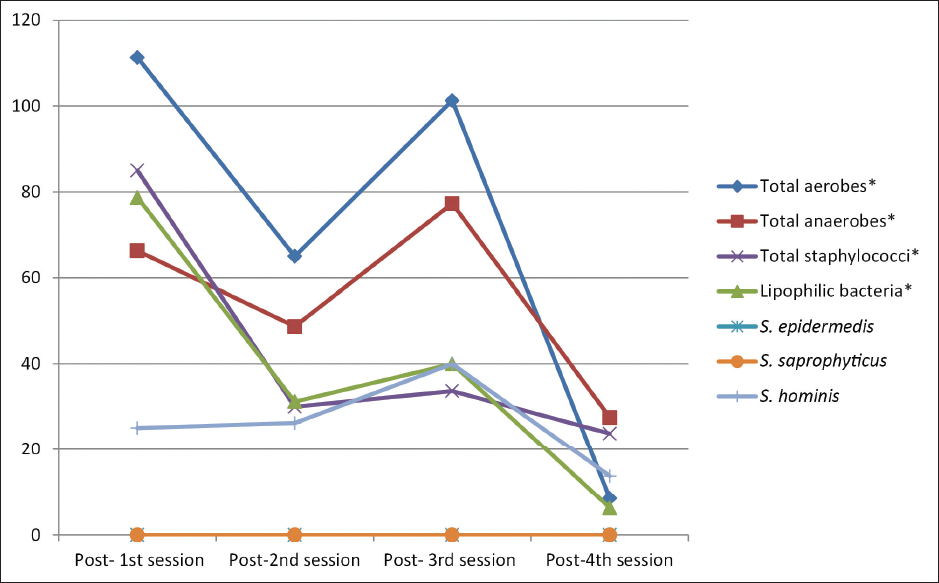

- Distribution of the studied participants (n = 30) according to the median bacterial colony count after each session (S1, S2, S3 and S4).
Subjective changes in sweat odour and amount
By the end of the study, twelve participants (40%) reported worsened sweat odour, two (6.7%) reported improved odour, and sixteen (53.3%) reported no change in odour [Table 3]. The predominant species among those with worsened and improved sweat odour was S.hominis. One participant who yielded S.aureus transiently in her axillary swabs reported a worsened axillary odour. As regards sweat amount, sixteen females (53.3%) reported no change, ten (33.3%) reported an increased amount and four (13.4%) reported decreased sweating.
| n | % | |
|---|---|---|
| Odour of sweat | ||
| Worsened | 12 | 40.0 |
| Improved | 2 | 6.7 |
| Unchanged | 16 | 53.3 |
| Amount of sweat | ||
| Decreased | 4 | 13.4 |
| Increased | 10 | 33.3 |
| Unchanged | 16 | 53.3 |
The baseline bacterial counts before the first laser session were not significantly different in subjects who reported worsened sweat odour later than in those who reported no change in odour (data not shown). However, counts prior to the fourth laser session showed marked differences, where statistically significant lower counts of total aerobes (52.5 versus 142.5 × 105 CFU/cm2) (p = 0.029), total anaerobes (23.7 versus 128.7 × 105 CFU/cm2) (p = 0.047) and S. hominis (17.5 versus 117.5 × 105 CFU/cm2) (p = 0.033) were found among those with worsened odour, indicating that lower counts of these organisms were associated with a perceived malodour of sweat [Table 4]. Since only two participants reported improved sweat odour, they were excluded from the statistical analysis correlating sweat odour and bacterial counts [Table 4]. These two participants had median bacterial counts (CFU/cm2) before their first and fourth laser sessions respectively, as follows: total aerobes (386.4 versus 391.3), total anaerobes (244.7 versus 1718.7 ×105) and S.hominis (301.2 versus 300 ×105 CFU/cm2).
| Type of bacteria | Colony count (×105 CFU/cm2) | p-value | |
|---|---|---|---|
| Worsened sweat odour | Unchanged sweat odour | ||
| Total aerobes | |||
| Mean ± SD | 102.5 ± 136.8 | 197.9 ± 158.3 | p = 0.029* U = 142.5 |
| Median | 52.5 | 142.5 | |
| Min-Max | 1.0–485.0 | 7.5–647.5 | |
| Total anaerobes | |||
| Mean ± SD | 97.4 ± 160.2 | 463.5 ± 1225.2 | p = 0.047* U = 138.5 |
| Median | 23.7 | 128.7 | |
| Min-Max | 52.0–572.5 | 2.5–5037.5 | |
| Total staphylococci | |||
| Mean ± SD | 72.1 ± 99.8 | 872.5 ± 2238.0 | p = 0.082 U = 133.5 |
| Median | 18.5 | 111.3 | |
| Min-Max | 1.5–310.0 | 2.5–8612.5 | |
| Lipophilic bacteria | |||
| Mean ± SD | 103.2 ± 110.1 | 205.3 ± 306.3 | p = 0.108.5 U = 1.980 |
| Median | 56.2 | 153.7 | |
| Min-Max | 0.0–342.5 | 0.0–1250.0 | |
| S.saprophyticus | |||
| Mean ± SD | 4.1 ± 11.5 | 24.2 ± 62.4 | p = 0.837 U = 101.0 |
| Median | 0.0 | 0.0 | |
| Min-Max | 0.0–37.5 | 0.0–237.5 | |
| S.hominis | |||
| Mean ± SD | 53.6 ± 73.1 | 198.8 ± 242.2 | p = 0.033* U = 148.0 |
| Median | 17.5 | 117.5 | |
| Min-Max | 0.0–242.5 | 2.5–825.0 | |
P*: p value significant at level <0.05
The twelve females who reported worsened sweat odour showed significant reductions in the following median bacterial counts (CFU/cm2) from their first to fourth sessions respectively (pre-session samples): total aerobes (248.7 versus 52.5 × 105, p = 0.005), total anaerobes (365 versus 23.7 × 105, p = 0.010), total staphylococci (232.5 versus 18.5 × 105, p = 0.008) and S.hominis (45 versus 17.5 × 105, p = 0.019). Further, there was an observed reduction, although statistically insignificant, in the median lipophilic bacterial count (307.5 versus 56.2 × 105). On the other hand, all the sixteen females who reported unchanged sweat odour had no significant difference in their bacterial counts [Table 5].
| Type of bacteria | Colony count (×105 CFU/cm2) | |||||
|---|---|---|---|---|---|---|
| Unchanged sweat odour (n = 16) | Worsened sweat odour (n = 12) | |||||
| Pre S1 | Pre S4 | p-value | Pre S1 | Pre S4 | p-value | |
| Total aerobes | ||||||
| Mean ± SD | 893.3 ± 1759.9 | 197.9 ± 158.3 | =0.163 Wilcoxon =41.0 | 690.2 ± 1052.6 | 102.5 ± 136.8 | = 0.005* Wilcoxon = 3.0 |
| Median | 253.4 | 142.5 | 248.7 | 52.5 | ||
| Min-Max | 40.0–6250.0 | 7.5–647.5 | 2.5–3817.5 | 1.0-485.0 | ||
| Total anaerobes | ||||||
| Mean ± SD | 343.0 ± 918.5 | 463.5 ± 1225.2 | = 0.196 Wilcoxon = 43.0 | 1121.6 ± 297.9 | 97.4 ± 160.2 | 0.010* Wilcoxon = 6.0 |
| Median | 311.2 | 128.7 | 365.0 | 23.7 | ||
| Min-Max | 3.9–1121.2 | 2.5–5037.5 | 2.5–6662.5 | 52.0–572.5 | ||
| Total Staphylococci | ||||||
| Mean ± SD | 344.3 ± 368.0 | 872.5 ± 2238.0 | = 0.214 Wilcoxon = 44.0 | 460.0 ± 746.1 | 72.1 ± 99.8 | 0.008* Wilcoxon = 5.0 |
| Median | 228.5 | 111.3 | 232.5 | 18.5 | ||
| Min-Max | 12.5–1397.5 | 2.5–8612.5 | 25.0–2720.0 | 1.5–310.0 | ||
| Lipophilic bacteria | ||||||
| Mean ± SD | 735.0 ± 2277.8 | 205.3 ± 306.3 | = 0.266 Wilcoxon = 46.5 | 1314.1 ± 2606.1 | 103.2 ± 110.1 | = 0.099 Wilcoxon = 15 |
| Median | 142.5 | 153.7 | 307.5 | 56.2 | ||
| Min-Max | 2.5–9262.3 | 0.0–1250.0 | 0.0–8287.0 | 0.0–342.5 | ||
| S.saprophyticus | ||||||
| Mean ± SD | 9.5 ± 22.7 | 24.2 ± 62.4 | 0.600 Wilcoxon = 13.0 | 0.42 ± 1.5 | 4.1 ± 11.5 | = 0.317 Wilcoxon = 1.000 |
| Median | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Min-Max | 0.0–79.7 | 0.0–237.5 | 0.0–5.0 | 0.0–37.5 | ||
| S.hominis | ||||||
| Mean ± SD | 238.6 ± 325.5 | 198.8 ± 242.2 | 0.642 Wilcoxon = 59.0 | 232.1 ± 308.9 | 53.6 ± 73.1 | = 0.019* Wilcoxon = 9.0 |
| Median | 107.5 | 117.5 | 45.0 | 17.5 | ||
| Min-Max | 7.5–1283.7 | 2.5–825.0 | 3.4–952.5 | 0.0–242.5 | ||
*p value significant at level <0.05*, S1: first session of Nd: YAG laser, S4: fourth session of Nd: YAG laser
There was a statistically significant positive correlation between sweat odour and total aerobic count (r = 0.433, p = 0.017), total anaerobic count (r = 0.377, p = 0.040), total staphylococci (r = 0.383, p = 0.036) and S.hominis (p = 0.497, p = 0.005) i.e., higher the bacterial counts, higher the likelihood of normal sweat odour, and vice versa. Lipophilic bacteria and S.saprophyticus were not correlated with sweat odour [Table 6].
| Bacterial parameter | rs | p value |
|---|---|---|
| Total aerobes | 0.433 | 0.017* |
| Total anaerobes | 0.377 | 0.040* |
| Total staphylococci | 0.383 | 0.036* |
| Lipophilic bacteria | 0.163 | 0.390 |
| S.saprophyticus | 0.301 | 0.105 |
| S.hominis | 0.497 | 0.005* |
rs: Spearman rho correlation co efficient
*: p value significant at level <0.05
Five participants gave a history of repeated folliculitis in their axillae before having their laser sessions; all five of them reported an improvement in this problem by the end of the study. On assessing the bacterial counts in these five participants, all median bacterial counts were found to be reduced. However, a significance test could not be performed due to the small number.
Discussion
There have been no previous studies evaluating the effect of Nd:YAG laser on skin flora and pathogens in vivo. Studying the antibacterial effect of Nd:YAG laser has important implications, firstly due to its widespread use for hair removal. Secondly, the antibacterial effect of Nd:YAG laser could be used for skin decolonization; this is a potential use not hitherto suggested, to our knowledge. Skin decolonization aims to reduce the bacterial load on the skin preoperatively, preventing surgical site infections that occur when high bacterial loads are present. Important pathogens implicated in such infections are S. aureus, especially MRSA. The results of our study might help develop new decolonization strategies, especially in the era of multidrug resistance. The degree of reduction of bacterial load either in the pre- versus post session counts or in the pre-S versus pre-S4 counts was found to be variable among participants (50%–70%). This variation among participants might be attributed to personal factors such as the composition and quantity of sebaceous secretions as well as the bacterial virulence factors which are strain-specific and may contribute to re-growth of bacteria. The extent to which bacterial reduction might be beneficial in alleviating sweat odour requires further studies, as the beneficial role of skin flora requires adequate balance of their counts.
Studies have reported the dominance of Staphylococci over other aerobic axillary flora (aerobic coryneforms and micrococci), especially among females.10,11 Staphylococci were thus selected for our study as an important axillary microbial flora since our participants were all females.
In the present study, the baseline median total aerobic bacterial count was 778.3 ± 1429.7 × 105 CFU/cm2, which is higher than that reported by Fazel et al (179.7 × 105 CFU/cm2).12 In the present study, the most predominant staphylococcal species was S. hominis followed by S. Saprophyticus and S. epidermidis. In line with our findings, Kloos & Musselwhite13 reported that the most abundant species on axillary skin was S. hominis. However, Fazel et al.12 reported that the predominant baseline bacteria were S. aureus followed by S. epidermidis and then S. saprophyticus. These differences might be due to differences in study populations including age, personal habits and precautions taken before sample collection. Variations in laboratory media and techniques used might also have contributed to these differences.
In the present study, when comparing median bacterial counts of pre-session versus post-session bacterial counts, there was a significant reduction of colony counts of total aerobes, total anaerobes, lipophilic bacteria, total staphylococci, and S.hominis. This finding was consistently seen in the four laser sessions, where post-session colony counts were significantly lower than pre-session ones. No similar significant reduction was seen in counts of S. epidermidis, S. saprophyticus, and S. aureus. This is probably owing to the lower colony counts of these species isolated in our study. The immediate antibacterial effect of Nd:YAG laser has also been reported in an in vitro study on S. aureus and Pseudomonas aeruginosa (after 24 hours).14
A delayed antibacterial effect of Nd:YAG laser was documented in our study, with reductions in total aerobic, anaerobic, staphylococcal, and S.hominis counts on comparing samples obtained before the first and fourth sessions. There was a significant immediate, but not delayed, inhibitory effect of the Nd:YAG laser on the lipophilic bacterial count as well. This might be due to variations in bacterial susceptibility to laser and the ability of some species to recover better than others. It was noted that there was no significant difference between counts of the second and third sessions while the difference was significant between the first and fourth sessions, indicating that the bacterial delayed inhibitory effect of Nd:YAG laser requires at least four sessions to become significant. Fazel et al.12 reported a significant reduction in the mean bacterial colony in the axillary region of laser participants after the sixth session of hair removal.
In more than half the participants (50%–96.3%), and in all laser sessions, the percentage of reduction of bacterial counts between each pre- and post-session reached ≥50% (except for the anaerobic count of the 2nd session). The most frequent rates of bacterial reduction was noted in counts of lipophilic bacteria, where 55.2%–96.3% of participants had ≥50% reduction in their pre-versus post session counts, in all four laser sessions, p = 0.003) (Table S1).
Concerning the delayed inhibitory effect of laser (pre-S versus pre-S4), ≥50% reduction of counts was seen in 50%–70% of participants in different bacterial parameters.
The antibacterial effect of Nd:YAG laser can explain the clearance of repeated folliculitis reported by five participants in the present study. Staphylococci, especially S. aureus, are the most frequent cause of bacterial folliculitis, while less commonly implicated genera include Streptococcus, Pseudomonas, and coliform bacteria.15 On assessing the median bacterial count in these five participants, all bacterial counts done were reduced, but a test of significance could not be performed due to the small number. Reduction of Staphylococci (including S. aureus) might explain the improvement of folliculitis following Nd:YAG laser sessions.
Possible explanations for the antibacterial effect of laser include (a) suppression of DNA metabolism and cell division, leading to degenerative changes up to cell pyknosis. There are three-phase responses of bacteria to laser irradiation: phase 1, bacteria inhibited but viable; phase 2, bacteria non-viable but intact; and phase 3, physical dissolution of bacteria,16 (b) irradiated endogenous bacterial photosensitiser resulting in induction of reactive oxygen species,17 (c) modulation of transmembrane convection directly by laser,18 (d) For pigmented bacteria, absorption of laser light by the pigments inside the bacteria leads to vaporization of water and cell lysis,19 (e) stimulatory effect on human lymphocytes.20
Side-effects of laser hair removal include pain, erythema, edema, dyschromia, blistering, erosions, or purpura.1 Changes in sweating have been reported but not thoroughly investigated. In the present study, most females reported either no change in sweat odour (53.3%) or worsened odour (40%). Only two females (6.7%) reported improvement in odour. In the study by Fazel et al.12 most (63.3%) participants who underwent six sessions of alexandrite laser treatment reported improved sweat odour; an unchanged odour was reported in 20%, and the odour was exacerbated in 16.7%. The differences in this respect between the studies may be attributed to several factors including differences in laser machines, different laser parameters used, number of sessions, and ethnic and individual variations.
Laser-induced changes in sweat odour maybe due to personal factors (such as the amount and composition of sweat, personal hygiene habits, and genetic factors) as well as the interplay of bacterial species by antagonism or synergism. In the present study, a significant correlation was noted between worsened sweat odour and lower counts of total aerobes (Figures already in results section), total anaerobes, total staphylococci and S.hominis. Our findings contradict those of Troccaz et al.,21 who reported that sweat odour improved with decreased axillary bacterial counts of S. hominis and higher counts of S. epidermidis. In contrast, Rennie et al.22 reported a lack of association between the total bacterial count of staphylococci and axillary odour, whereas coryneform bacteria had a statistically significant association with odour intensity.22 Fazel et al.12 reported that S.epidermidis was predominant in those with improved odour, Micrococcus luteus in those with worsened odour and S. aureus in those with unchanged odour. In contrast, in our study, S.hominis was predominant in all participants regardless of their sweat odour. Troccaz et al.21 also found that offensive body odour was linked to higher proportions of Corynebacterium, Anaerococcus, Peptoniphilus, and S. hominis, possibly due to their production of malodorous volatiles from apocrine sweat. In the present study, although higher numbers of aerobes, anaerobes and staphylococci were associated with better sweat odour in contrast to most other studies, it is possible that other untested bacterial species might have decreased simultaneously contributing to an improved sweat odour in our subjects. The association of certain species with improved odour has been investigated in some studies as a therapeutic option for malodour, with axillary bacterial transplantation where the malodor-causing microbiome is replaced with a healthy non-odorous axillary microbiome.23 Transplantation of axillary microbiome has been performed in only a few studies as clinical trials to improve axillary odour. To the best of our knowledge, these studies did not report side effects following transplantation of microbiome, whether these bacteria were transplanted from the same person but from a different body site (homologous), or from the axillary skin of a different person. Further studies in the future might give us more evidence of the safety of this transplantation, and the occurrence of any possible side effects which might include hypersensitivity or, possibly, gradual disappearance of the transplanted microbiome and replacement by the primary undesired one.
Females who reported unchanged sweat odour revealed no significant differences in the median bacterial counts before and after laser. However, laser might have a species-dependent effect leading to dysbiosis, with some species increasing relative to others, as reported by Troccaz et al.21 and Fazel et al.12 In our study however, dysbiosis could not be proved in relation to other species, since only staphylococci were studied.
In our study, 53.3% of females reported no change in sweat amount, 33.3% reported increased amount while 13.4% reported decreased sweating. Increased axillary sweating induced by 1064 nm Nd:YAG laser was studied by Aydin et al.24 after 6–10 monthly sessions of laser hair removal (10-mm spot size, 30–60 J/cm fluence and 55–65 ms pulse width). This agrees with some reports on induction of sympathetic skin response after laser stimulation25,26 On the other hand, reduction of axillary sweating by 1064 nm Nd:YAG for treatment of bromidrosis acting subdermally rather than transdermally. The laser reaches its target directly resulting in histopathologic alterations in eccrine glands with effective reduction of hyperhidrotic activity. They also suggest that heating affects the surrounding nerve fibres contributing to the clinical effect.27 Along with our results, similar findings were reported with diode and alexandrite laser for axillary hair removal and even inguinal hyperhidrosis following inguinal laser hair removal.28–30 From previous data, one can speculate that 1064 nm Nd:YAG laser may have both stimulatory effect on axillary sweating when applied transdermally and inhibitory effect when applied subdermally. According to Helou et al, the mean number of laser sessions needed for onset of hyperhidrosis was 6, with a minimum of 3 sessions and a maximum of 11 sessions.29
The underlying mechanism for excessive sweating after the laser is still not fully understood. The laser may induce direct thermal injury or indirect stimulation via nerve fibres to sweat glands close to the hair follicles. Devices operating in the near-infrared wavelength region penetrate deeply into the dermis and even the subcutaneous tissue targeting the heavily pigmented matrix of the hair. Therefore, the deep-going energy could activate the sweat glands lying near the deep terminal hair bulbs of the axilla whenthe laser is applied through the skin surface. This hyperactive state is transient in 76.7%.29 This is possible through the influence of periglandular temperature on glandular metabolism, neurotransmitter release and metabolism, membrane transport, and receptor affinity.30
On the contrary, Letada et al.31 reported a significant reduction in axillary sweating after five sessions of 1064 nm Nd:YAG laser. Histologic analysis of axillary tissue did not demonstrate any notable change between pre- and post-treatment. This led Letada et al.31 to conclude that the mechanism of action might involve cellular or subcellular alterations through the heating of melanin or water chromophores, thus impacting the sympathetic cholinergic transmissions in the axilla.32
Limitations of the present study included a small sample size, few laser sessions, a short follow-up period, and subjective assessment of sweat odour and quantity; only limited bacterial species were included in the study. Lack of control group, only woman study subjects and inclusion of only axillary site are other limitations.
Conclusion
Nd:YAG laser (1064 nm) may have an antibacterial effect against certain bacterial species. Both immediate and delayed antibacterial effects were observed, but longer follow-up is required to evaluate the duration of these effects. Nd:YAG laser may also affect sweat odour by altering the local skin microbiome.
Declaration of patient consent
The authorsall appropriate patient consent
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Supplementary File 1 DownloadReferences
- Tanzi EL, Alster TS. Long-pulsed 1064-nm Nd:YAG laser-assisted hair removal in all skin types. Dermatol Surg. 2004;30:13-7.
- [CrossRef] [PubMed] [Google Scholar]
- Kang CN, Shah M, Lynde C, Fleming P. Hair removal practices: A literature review. Skin Therapy Lett. 2021;26:6-11.
- [Google Scholar]
- Lask G, Elman M, Slatkine M, Waldman A, Rozenberg Z. Laser-assisted hair removal by selective photothermolysis. Preliminary results. Dermatol Surg. 1997;23:737-9.
- [CrossRef] [PubMed] [Google Scholar]
- Lin TY, Manuskiatti W, Dierickx CC, Farinelli WA, Fisher ME, Flotte T, et al. Hair growth cycle affects hair follicle destruction by ruby laser pulses. J Invest Dermatol. 1998;111:107-13.
- [CrossRef] [PubMed] [Google Scholar]
- Russ D, Kienle A, Falkenstein W, Steiner R. Optimierung der Laserepilation durch Simulation der thermischen Wirkung der Laserstrahlung. Laser-Medizin: eine interdisziplinäre Zeitschrift; Praxis, Klinik, Forschung. 2000;15:87-95.
- [PubMed] [Google Scholar]
- James AG, Austin CJ, Cox DS, Taylor D, Calvert R. Microbiological and biochemical origins of human axillary odour. FEMS Microbiol Ecol. 2013;83:527-40.
- [CrossRef] [PubMed] [Google Scholar]
- Herbenick D, Hensel D, Smith NK, Schick V, Reece M, Sanders SA, et al. Pubic hair removal and sexual behavior: findings from a prospective daily diary study of sexually active women in the United States. J Sex Med. 2013;10:678-85.
- [CrossRef] [PubMed] [Google Scholar]
- Selwyn S, Ellis H. Skin bacteria and skin disinfection reconsidered. Br Med J. 1972;1:136-40.
- [CrossRef] [PubMed] [Google Scholar]
- Condalab. Staphylococcus Chromogenic Agar. Available from: https://www.condalab.com/int/en/culture-media-for-clinical-diagnosis/1311-14886-staphylococcus-chromogenic-agar.html. [Accessed in: Aug, 2022].
- Callewaert C, Kerckhof FM, Granitsiotis MS, Van Gele M, Van de Wiele T, Boon N. Characterization of staphylococcus and corynebacterium clusters in the human axillary region. PLoS One. 2013;8:e70538.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Taylor D, Daulby A, Grimshaw S, James G, Mercer J, Vaziri S. Characterization of the microflora of the human axilla. Int J Cosmet Sci. 2003;25:137-45.
- [CrossRef] [PubMed] [Google Scholar]
- Fazel Z, Majidpour A, Behrangi E, Fathizadeh S, Nokandeh M, Atefi N, et al. Using the hair removal laser in the axillary region and its effect on normal microbial flora. J Lasers Med Sci. 2020;11:255-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kloos WE, Musselwhite MS. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975;30:381-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ebid AA, Alhammad RM, Alhindi RT, Alghamdi AA, Alqarhi AK, Abdullah HA, et al. Effect of high-power Nd:YAG laser on the growth of Staphylococcus aureus and Pseudomonas aeruginosa: An experimental study. J Phys Ther Sci. 2021;33:222-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-10.
- [CrossRef] [PubMed] [Google Scholar]
- Yuan X, Song Y, Song Y, Xu J, Wu Y, Glidle A, et al. Effect of laser irradiation on cell function and its implications in raman spectroscopy. Appl Environ Microbiol. 2018;84:e02508-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Seyedmousavi S, Hashemi SJ, Rezaie S, Fateh M, Djavid GE, Zibafar E, et al. Effects of low-level laser irradiation on the pathogenicity of Candida albicans: in vitro and in vivo study. Photomed Laser Surg. 2014;32:322-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sommer AP, Zhu D, Scharnweber T. Laser modulated transmembrane convection: Implementation in cancer chemotherapy. J Control Release. 2010;148:131-4.
- [CrossRef] [PubMed] [Google Scholar]
- Gokhale S, Padhye A, Sumanth S. Bactericidal effect of Nd: YAG laser in an in vitro tissue model—a light microscopic evaluation. J Oral Laser Appl. 2010;10:17.
- [Google Scholar]
- Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA. Low-level laser therapy in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 2006;24:158-68.
- [CrossRef] [PubMed] [Google Scholar]
- Troccaz M, Gaïa N, Beccucci S, Schrenzel J, Cayeux I, Starkenmann C, et al. Mapping axillary microbiota responsible for body odours using a culture-independent approach. Microbiome. 2015;3:3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rennie PJ, Gower DB, Holland KT, Mallet AI, Watkins WJ. The skin microflora and the formation of human axillary odour. Int J Cosmet Sci. 1990;12:197-207.
- [CrossRef] [PubMed] [Google Scholar]
- Callewaert C, Lambert J, Van de Wiele T. Towards a bacterial treatment for armpit malodour. Exp Dermatol. 2017;26:388-91.
- [CrossRef] [PubMed] [Google Scholar]
- Aydin F, Pancar GS, Senturk N, Bek Y, Yuksel EP, Canturk T, et al. Axillary hair removal with 1064-nm Nd:YAG laser increases sweat production. Clin Exp Dermatol. 2010;35:588-92.
- [CrossRef] [PubMed] [Google Scholar]
- Cervera A, Veciana M, Valls-Solé J. Sympathetic sudomotor skin responses induced by laser stimuli in normal human subjects. Neurosci Lett. 2002;334:115-8.
- [CrossRef] [PubMed] [Google Scholar]
- Rossi P, Truini A, Serrao M, Iannetti GD, Parisi L, Pozzessere G, et al. Sympathetic skin response evoked by laser skin stimulation. Funct Neurol. 2002;17:129-32.
- [PubMed] [Google Scholar]
- Goldman A, Wollina U. Subdermal Nd:YAG laser for axillary hyperhidrosis. Dermatol Surg. 2008;34:756-62.
- [CrossRef] [PubMed] [Google Scholar]
- Hélou J, Soutou B, Jamous R, Tomb R. Nouveaux effets indésirables du laser dépilatoire axillaire [Novel adverse effects of laser-assisted axillary hair removal] Annales de Dermatologie et de Vénéréologie. 2009;136:495-500.
- [PubMed] [Google Scholar]
- Helou J, Habre M, Soutou B, Maatouk I, Ibrahim T, Tomb R. Reversibility of hyperhidrosis post axillary depilatory laser. Lasers Med Sci. 2014;29:717-21.
- [CrossRef] [PubMed] [Google Scholar]
- Obeid G, Helou J, Maatouk I, Moutran R, Tomb R. Depilatory laser: a potential causative factor for inguinal hyperhidrosis: Report of three cases. J Cosmet Laser Ther. 2013;15:286-9.
- [CrossRef] [PubMed] [Google Scholar]
- Letada PR, Landers JT, Uebelhoer NS, Shumaker PR. Treatment of focal axillary hyperhidrosis using a long-pulsed Nd:YAG 1064 nm laser at hair reduction settings. J Drugs Dermatol. 2012;11:59-63.
- [PubMed] [Google Scholar]
- Cervantes J, Perper M, Eber AE, Fertig RM, Tsatalis JP, Nouri K. Laser treatment of primary axillary hyperhidrosis: A review of the literature. Lasers Med Sci. 2018;33:675-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
-
Sections -
Statistics -
Download
- Introduction
- Subjects and Methods
- Laser sessions
- Sample collection
- Laboratory methods
- Statistical analysis
- Results
- The immediate antimicrobial effect of Nd:YAG laser
- The delayed antimicrobial effect of Nd:YAG laser
- Subjective changes in sweat odour and amount
- Discussion
- Conclusion
Suggested read for related articles:
- April 26, 2023
- February 21, 2024
- August 23, 2024




































